Sensitivity of Extracellular Vesicles from Human Blood Serum to Various Detergents
1Institute of Higher Nervous Activity and Neurophysiology RAS,
5A Butlerova str., Moscow, 117485 Russia; *e-mail: al_yakovlev@ihna.ru
2Scientific and Practical Psychoneurological Center named after Z.P. Solovy’ov DZM,
43 Donskaya str., Moscow, 115419 Russia
Keywords:exosomes; microvesicles; blood serum; dynamic light scattering; detergents
DOI:10.18097/BMCRM00143
Blood exosomes and microvesicles, collectively known as small extracellular vesicles (sEV), are vesicles about 100-150 nm in size. Small EV are involved in various aspects of signaling in the body; in addition, they can serve as markers of various pathologies. For biochemical studies, vesicle solubilization is often required. We tested the ability of various detergents to dissolve membranes of the sEV. Small EV were isolated from the blood serum of healthy volunteers by gel filtration on Sepharose CL-2B and tried to solubilize them using the anionic detergent DOC (sodium deoxycholate), non-ionic detergent Brij 35 (polyoxyethyleneglycol dodecyl ether), zwitterionic detergent CHAPS (3 - [(3-chloramidopropyl) dimethylammonio] -1-propanesulfonate), and cationic detergent CTAB (cetyl trimethylammonium bromide). The concentration of sEV in the solution was determined by dynamic light scattering. We find DOC is the most effective for sEV solubilization.
The List of Abbreviations:PBS, phosphate-buffered saline; SDS, sodium dodecyl sulfate; DOC, sodium deoxycholate; Brij 35, polyethylene glycol dodecyl ether; CHAPS, 3-[(3-chloramidopropyl) dimethylammonio]-1-propanesulfonate; CTAB, cetyl-trimethylammonium bromide; Triton X-100, tert-octyl phenoxypolyethoxyethanol; NP-40, octyl phenoxypolyethoxyethanol; EV, extracellular vesicles.
INTRODUCTION
Small EV (sEV) are membrane vesicles secreted by almost all cells of the body [1]. It has been shown that sEV contain nucleic acids, enzymes, receptors, and other molecules, but a detailed analysis of the composition of sEV has not yet been performed. The size of exosomes is 50-150 nm, while the size of microvesicles, another class of EV, is 100-200 nm [2]. Studying sEV, it is often necessary to solubilize the vesicle membrane and examine the soluble fraction. For example, one can sequentially biotinylate first the surface proteins of the sEV and then solubilize the membrane and to biotinylate the intravesicular proteins with another reagent [3]. This provides a more detailed characterization of the sEV composition. Moreover, using the selective biotinylation approach it is possible to detect markers of various diseases [4]. It is known that sEV exhibit selective sensitivity to different detergents [5]. According to published data, 0.125% SDS, 0.075% Triton X-100 or 0.1% DOC can solubilize both microvesicles and exosomes [5]. However, most previous results have been obtained using sEV from cell culture fluids cultures, and sensitivity of serum sEV to detergents remains unknown. Some studies have used 3% Triton X-100 for lysis of plasma sEV (without clear justification) [6]. SDS, Triton X-100, and NP-40 detergents, each at a concentration of 1%, have been used for lysis of saliva sEV; however, nonionic detergents could not provide complete particle destruction [7]. In addition, even for the sensitivity of sEV isolated from culture fluid after cell cultivation not everything is clear in the context of their sensitivity to detergents. Some authors believe that, at least nonionic detergents do not solubilize the sEV membrane, but rather reduce the zeta potential of the particles and thus reduce the colloidal stability of the particles in solution, which can lead, for example, to the precipitation of the particles [8]. In some works, a deliberately high concentration of detergents, (e.g. 2% SDS) was used to solubilize sEV from cell cultures [9]. According to some data, sEV isolated from blood plasma can be destroyed by 1% Triton X-100, although the use of different methods of mEV isolation leads to the release of particles which differ significantly in their sensitivity to the detergent [10]. Lysis of sEV from culture fluid performed in a mixture of detergents 1% NP-40, 1% DOC and 0.1% SDS increases the number of extracted proteins compared to lysis of sEV in 1% Triton X-100 [11].
Thus, to date, there are no clear data on the sensitivity of sEV to detergents, much less sEV from human serum. Nevertheless, such data are now in demand for a number of biochemical experiments. We need to know the sensitivity of sEV to detergents for planning experiments to determine the activity of intravesicular enzymes. We have selected the main detergents frequently used in the laboratory and tested the sensitivity of sEV to them. We have found that DOC was the most effective for sEV solubilization.
MATERIALS AND METHODS
Phosphate-salt buffer (PBS, in tablets, «Biolot», Russia), sodium dodecyl sulfate (SDS, Serva, Germany), sodium deoxycholate (DOC, «Calbiochem», USA) dodecyl ether of polyethylene glycol (Brij 35, MP Biomedicals, USA), 3-[(3-chloramidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS, «MP Biomedicals»), cetyl trimethylammonium bromide (CTAB, «Fluka», Germany), CL-2B Sepharose («GE Healthcare», USA) were used in the experiments. All solutions were prepared in double deionized water with a resistance of 18.2 MOhm x cm.
Materials for the study
Human blood serum was the material for the study. Blood sampling in healthy volunteers was performed from the ulnar vein in the morning hours on an empty stomach. Many factors are known to influence the EV formation [12], so the blood sampling procedure and the preanalytical stage of sample preparation were standardized as much as possible. The same conditions were determined for all samples, namely: time (not more than 30 min) and method of blood sampling; the temperature in room between blood sampling and centrifugation (22-23°C); centrifugation conditions; conditions of biological material storage at all stages of the analysis.
Extraction of sEV from human serum
Serum samples were obtained and purified from cell residues and high molecular weight complexes by centrifugation at 4°C for 30 min at 10000 g. The column was filled with 10 mL of Sepharose CL-2B resin and equilibrated with PBS. Pre-centrifuged serum was applied to the column in a volume of 500 μL, 2.5 mL of PBS was applied after adding the sample, and the eluate was discarded. After that, 1 mL of PBS was applied and the eluate was collected for subsequent analysis. Before another assay, the column was washed with at least 15 mL of PBS [13]. The isolated sEV were stored at -80°C.
Processing of sEV with detergents
Detergents were added to the sEV samples up to a concentration of 1% from a freshly prepared 20% stock solution. Solutions of all detergents were prepared with PBS, which was also used as a blank sample. After 5 min of mixing, the sEV concentration was determined using dynamic light scattering. All operations were performed at room temperature.
Determination of sEV concentration using dynamic light scattering
The intensity of dynamic light scattering of exosomes was determined using a Zetasizer Nano S instrument («Malvern Panalytical», UK). A quartz cuvette was filled with sEV in the presence of a given concentration of detergent and the light scattering was determined at an angle of 173 degrees at a wavelength of 633 nm in four repetitions of 50 s each. The equipment makes it possible to calculate the size of nanoparticles, as well as to determine the number of photons scattered on the particles, expressed in thousands of photons per second (kcps). The total number of photons scattered on the particles serves as a parameter reflecting the concentration of sEV in the sample [14].
Statistical processing of the results
Statistical analysis was performed using Graphpad Prism ver. 8.0. The results are presented as mean ± standard deviation. Nonparametric correlation analysis was used to determine the relationship between the variables; Spearman correlation coefficient and 95% confidence interval were presented. Sixteen samples were used for the experiment to determine the sensitivity of sEV to different detergents, and 10 samples each were used for the other experiments.
RESULTS
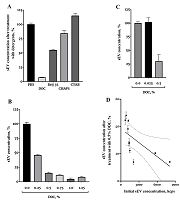
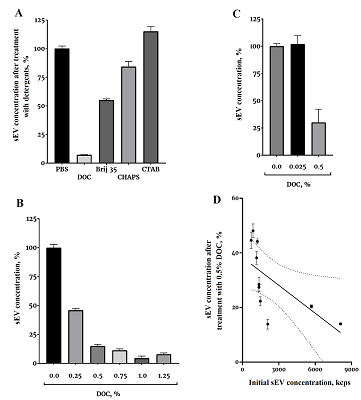
In the first experiment, a significantly different sensitivity of the sEV to the detergents was demonstrated (Fig. 1A). All detergents were used at a concentration of 1%, which significantly exceeded the critical micelle concentration for each detergent. The effect on sEV solubilization was tested for two other detergents, SDS and Triton X-100, but the 1% solution of these detergents in PBS had too high light scattering, so that it was not possible to examine any objects in it using dynamic light scattering (data not shown). Among the detergents studied, DOC showed the highest efficiency, so in the subsequent experiment the dependence of the solubilization efficiency on the DOC concentration was investigated (Fig. 1B). We see that effective solubilization can be achieved at a DOC concentration of 0.5%. Efficiency of solubilization with 0.5% DOC was further evaluated in the following experiment using samples with significantly different sEV counts (Fig. 1B). On average, 0.5% DOC solubilized 75% of the vesicles. The fraction of sEV solubilized by 0.5% DOC depended on the initial concentration of sEV in the sample (Fig. 1G). The higher the concentration of sEV, the more effective the solubilization was. The Spearman correlation coefficient was -0.93, p<0.0003. The coefficient of determination for this approximation of 0.42 was reasonably good.
DISCUSSION
This work solved a rather important experimental problem - determination of the sensitivity of sEV to detergents. The task of sEV solubilization regularly arises in studies. For example, separation of sEV into membrane and soluble fractions is required to study intravesicular enzymatic activity. In this work, we showed that the anionic detergent DOC at relatively low concentrations can effectively solublize the sEV membrane. The nonionic determgent Brij 35 at a concentration of 1% soluilized about half of the vesicles (Fig. 1A), which could be probably be suitable for certain experimental tasks. The zwitterionic detergent CHAPS was virtually ineffective for sEV solubilization, and the cationic detergent CTAB was not suitable for sEV solubilization at all. For some analytical methods, the anionic detergent DOC is not applicable because it significantly denatures proteins; in such case, Brij 35, can be considered as an alternative for the experiment. One of the directions for further research is the search for sEV subfractions that are selectively sensitive to different detergents. There are already works on cell cultures [5], now we can repeat the experiments on blood sEV, bearing in mind that blood sEV are much more resistant to the action of detergents than sEV from the culture fluid (Fig. 1A). However, even though they are more resistant to detergents compared to culture, blood sEV can also have different sensitivity to detergents. For example, exosomes are more resistant to many detergents than microvesicles [5]. The work shows a reproducible result of the action of 0.5% DOC on samples with different concentrations of sEV (Fig. 1c), but with a clear dependence of the solubilization efficiency on the concentration of sEV (Fig. 1G). Solubilization is most effective when the initial concentration of sEV is high.
ACKNOWLEDGEMENTS
The authors express their sincere gratitude to the Russian representative office of Malvern Panalytical and personally to S.L. Vasin and E.O. Dulyakin for their help in developing methods of vesicle registration using dynamic light scattering.
FUNDING
The work was carried out within the topic AAAA-A19-119071990046-9 «Neurogenetics» of the State Assignment of the Institute of Higher Nervous Activity and Neurophysiology RAS.
COMPLIANCE WITH ETHICAL STANDARDS
All procedures performed in the study with human participants were approved by the local ethical committee of the Z.P. Solovy’ov Scientific and Practical Psychoneurological Center (no. 42 of August 23, 2019). Informed voluntary consent was obtained from each of the participants included in the study.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
SUPPLEMENTARY
Supplementary materials are available at http://dx.doi.org/10.18097/BMCRM00143
REFERENCES
- Bavisotto, C. C., Scalia, F., Gammazza, A. M., Carlisi, D., Bucchieri, F., de Macario, E. C., Macario, A. J. L., Cappello, F., Campanella, C. (2019) Extracellular Vesicle-Mediated Cell-Cell Communication in the Nervous System: Focus on Neurological Diseases. Int. J. Mol. Sci., 20(2), Article 434. DOI
- Raposo, G., Stoorvogel, W. (2013) Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol., 200(4), 373-383. DOI
- Diaz, G., Wolfe, L. M., Kruh-Garcia, N. A., Dobos, K. M. (2016) Changes in the Membrane-Associated Proteins of Exosomes Released from Human Macrophages after Mycobacterium tuberculosis Infection. Sci. Rep., 6, Article 37975. DOI
- Castillo, J., Bernard, V., San Lucas, F. A., Allenson, K., Capello, M., Kim, D. U., Gascoyne, P., Mulu, F. C., Stephens, B. M., Huang, J., Wang, H., Momin, A. A., Jacamo, R. O., Katz, M., Wolff, R., Javle, M., Varadhachary, G., Wistuba, II, Hanash, S., Maitra, A., Alvarez, H. (2018) Surfaceome profiling enables isolation of cancer-specific exosomal cargo in liquid biopsies from pancreatic cancer patients. Ann. Oncol., 29(1), 223-229. DOI
- Osteikoetxea, X., Sodar, B., Nemeth, A., Szabo-Taylor, K., Paloczi, K., Vukman, K. V., Tamasi, V., Balogh, A., Kittel, A., Pallinger, E., Buzas, E. I. (2015) Differential detergent sensitivity of extracellular vesicle subpopulations. Organic & Biomolecular Chemistry, 13(38), 9775-9782. DOI
- Martelli, F., Macera, L., Spezia, P. G., Medici, C., Pistello, M., Guasti, D., Romagnoli, P., Maggi, F., Giannecchini, S. (2018) Torquetenovirus detection in exosomes enriched vesicles circulating in human plasma samples. Virology Journal, 15, Article 145. DOI
- Kumeda, N., Ogawa, Y., Akimoto, Y., Kawakami, H., Tsujimoto, M., Yanoshita, R. (2017) Characterization of Membrane Integrity and Morphological Stability of Human Salivary Exosomes. Biol. Pharm. Bull., 40(8), 1183-1191. DOI
- Midekessa, G., Godakumara, K., Ord, J., Viil, J., Lattekivi, F., Dissanayake, K., Kopanchuk, S., Rinken, A., Andronowska, A., Bhattacharjee, S., Rinken, T., Fazeli, A. (2020) Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes that Determine Colloidal Stability. Acs Omega, 5(27), 16701-16710. DOI
- Cunnane, E. M., Lorentz, K. L., Ramaswamy, A. K., Gupta, P., Mandal, B. B., O'Brien, F. J., Weinbaum, J. S., Vorp, D. A. (2020). Extracellular Vesicles Enhance the Remodeling of Cell-Free Silk Vascular Scaffolds in Rat Aortae. Acs Applied Materials & Interfaces, 12(24), 26955-26965. DOI
- Tian, Y., Gong, M. F., Hu, Y. Y., Liu, H. S., Zhang, W. Q., Zhang, M. M., Hu, X. X., Aubert, D., Zhu, S. B., Wu, L., Yan, X. M. (2020) Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J. Extracell. Vesicles, 9(1), Article 1697028. DOI
- Subedi, P., Schneider, M., Philipp, J., Azimzadeh, O., Metzger, F., Moertl, S., Atkinson, M. J., Tapio, S. (2019) Comparison of methods to isolate proteins from extracellular vesicles for mass spectrometry-based proteomic analyses. Anal. Biochem., 584, Article 113390. DOI
- Witwer, K. W., Buzás, E. I., Bemis, L. T., Bora, A., Lässer, C., Lötvall, J., Nolte-‘t Hoen, E. N., Piper, M. G., Sivaraman, S., Skog, J., Théry, C., Wauben, M. H., Hochberg, F. (2013) Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles, 2(1), 20360. DOI
- Gamez-Valero, A., Monguio-Tortajada, M., Carreras-Planella, L., Marcel-la, F., Beyer, K., Borras, F. E. (2016) Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles' characteristics compared to precipitating agents. Sci. Rep., 6, Article 33641. DOI
- Yakovlev, A. A., Druzhkova, T. A., Nikolaev, R. V., Kuznetsova, V. E., Gruzdev, S. K., Guekht, A. B., Gulyaeva, N. V. (2019) Elevated Levels of Serum Exosomes in Patients with Major Depressive Disorder. Neurochemical Journal, 13(4), 385-390. DOI