Electrochemical Analysis of Metabolites as a Method for Cytochromes P450 Activity Determination
1Pirogov Russian National Research Medical University, 1 Ostrovityanova str. Moscow 117997, Russia;
*e-mail: v_shumyantseva@mail.ru
2Institute of Biomedical Chemistry, 10 Pogodinskaya str., Moscow, 119121 Russia
Keywords:cytochromes P450; drugs; metabolites; electrochemistry
DOI:10.18097/BMCRM00176
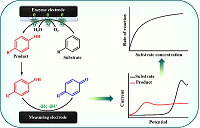
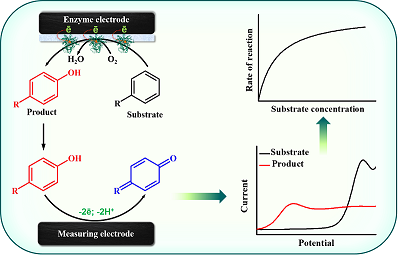
The review deals with the electrochemical methods for determination of metabolites of cytochromes P450 catalyzed reactions. We have focused on the electrochemical determination of metabolites of drugs and some endogenous compounds. We have reviewed bielectrode systems for determination of cytochrome P450 activity, where one electrode serves as a matrix for enzyme immobilization and a source of electrons for heme iron ion reduction and initialization of the catalytic reaction towards a substrate and the second one is being used for quantification of the products formed by their electrochemical oxidation. Such systems allow one to elude additional steps of separation of reaction substrates and products. The review also includes discussion of the ways to increase the analytical sensitivity and decrease the limit of detection of the investigated metabolites by chemical modification of electrodes. We demonstrate the possibilities of these systems for cytochrome P450 kinetics analysis and the perspectives of their further improvement, such as increasing the sensitivity of metabolite electrochemical determination by modern electrode modificators, including carbon-based, and construction of devices for automatic monitoring of the products.
|
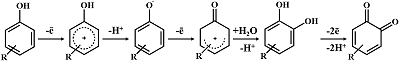
Figure 1.
The general mechanism of the electrochemical oxidation of hydroxyphenyl-containing compounds. Adapted from [58].
|
|
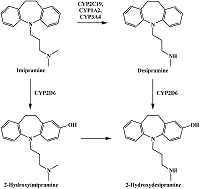
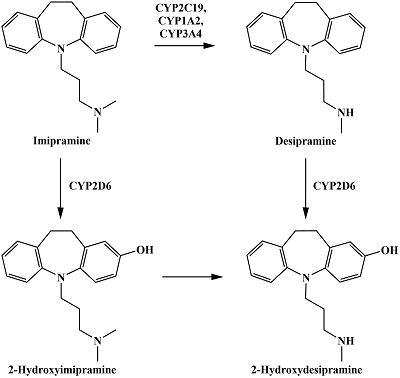
Figure 5.
The reactions of imipramine metabolism catalyzed by CYP2C19, CYP1A2, CYP3A4 and CYP2D6. Adapted from [23].
|
|
CLOSE

|
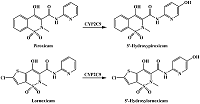
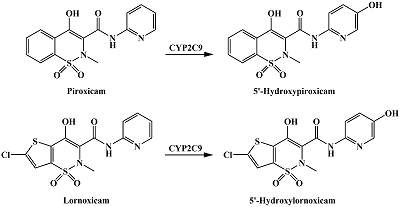
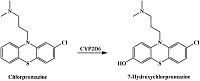
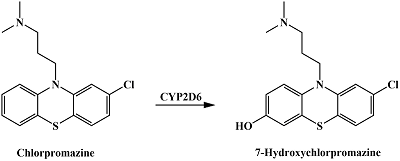
Table 1.
Catalyzed by CYPs reactions of drug metabolism resulting in hydroxyl group formation in aromatic rings.
|
FUNDING
- Manikandan, P., Nagini, S. (2018) Cytochrome P450 structure, function and clinical significance: a review. Curr. Drug Targets, 19(1), 38-54. DOI
- Lynch, T., Price, A. (2007) The effect of cytochrome p450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician, 76(3), 391-396.
- Baston, E., Leroux, F.R. (2007) Inhibitors of steroidal cytochrome p450 enzymes as targets for drug development. Recent Pat. Anticancer Drug Discov., 2(1), 31-58. DOI
- Hrycay, E.G., Bandiera, S.M. (2015) Monooxygenase, peroxidase and peroxygenase properties and reaction mechanisms of cytochrome p450 enzymes. in monooxygenase, peroxidase and peroxygenase properties and mechanisms of cytochrome P450 (E. Hrycay and S. Bandiera eds). Springer, Cham, pp. 1-61. DOI
- Zukiswa, J., Sandrine, L., Frederic N. (2018) Cardiovascular benefits of dietary melatonin: a myth or a reality? Frontiers in Physiology, 9, 528. DOI
- Protti, M., Mandrioli, R., Marasca, C., Cavalli, A., Serretti, A., Mercolini, L. (2020) New-generation, non-SSRI antidepressants: Drug-drug interactions and therapeutic drug monitoring. Part 2: NaSSAs, NRIs, SNDRIs, MASSAs, NDRIs, and others. Med. Res. Rev., 40, 1794-1832. DOI
- Cheng, Z.N., Shu, Y., Liu, Z.Q., Wang, L.S., Ou-Yang, D.S., Zhou, H.H. (2001) Role of cytochrome P450 in estradiol metabolism in vitro. Acta Pharmacol. Sin., 22(2), 148-154. DOI
- Ogburn, E.T., Jones, D.R., Masters, A.R., Xu, C., Guo, Y., Desta, Z. (2010) Efavirenz primary and secondary metabolism in vitro and in vivo: identification of novel metabolic pathways and cytochrome P450 2A6 as the principal catalyst of efavirenz 7-hydroxylation. Drug Metabolism and Disposition, 38(7), 1218-1229. DOI
- Restrepo, J.G., Garcia-Martin, E., Martinez, C., Agundez, J.A.G. (2009) Polymorphic drug metabolism in anaesthesia. Current Drug Metabolism, 10(3), 236-246. DOI
- Baldwin, S.J., Clarke, S.E., Chenery, R.J. (1999) Characterization of the cytochrome P450 enzymes involved in the in vitro metabolism of rosiglitazone. British Journal of Clinical Pharmacology, 48, 424-432. DOI
- Ufer, M. (2005) Comparative Pharmacokinetics of Vitamin K Antagonists. Clin. Pharmacokinet., 44, 1227-1246. DOI
- Bort, R., Macé, K., Boobis, A., Gómez-Lechón, M.-J., Pfeifer, A., Castell, J. (1999) Hepatic metabolism of diclofenac: role of human CYP in the minor oxidative pathways. Biochemical Pharmacology, 58(5), 787-796. DOI
- Kohl, C., Steinkellner, M. (2000) Prediction of pharmacokinetic drug/drug interactions from In vitro data: interactions of the nonsteroidal anti-inflammatory drug lornoxicam with oral anticoagulants. Drug Metab. Dispos., 28(2), 161-168. DOI
- Tracy, T.S., Hutzler, J.M., Haining, R.L., Rettie, A.E., Hummel, M.A., Dickmann, L.J. (2002) Polymorphic variants (CYP2C9*3 and CYP2C9*5) and the F114L Active site mutation of CYP2C9: effect on atypical kinetic metabolism profiles. Drug Metab. Dispos., 30(4), 385-390. DOI
- Yamanaka, H., Nakajima, M., Hara, Y., Katoh, M., Tachibana, O., Yamashita, J., Yokoi, T. (2005) Urinary excretion of phenytoin metabolites, 5-(4'-hydroxyphenyl)-5-phenylhydantoin and its O-glucuronide in humans and analysis of genetic polymorphisms of UDP-glucuronosyltransferases. Drug Metab. Pharmacokinet., 20(2), 135-143. DOI
- Vogl, S., Lutz, R.W., Schönfelder, G., Lutz, W.K. (2015) CYP2C9 Genotype vs. Metabolic Phenotype for Individual Drug Dosing—A Correlation Analysis Using Flurbiprofen as Probe Drug. PLOS ONE, 10(3), e0120403. DOI
- Piatkov, I., Rochester, C., Jones, T., Boyages, S. (2010) Warfarin Toxicity and Individual Variability—Clinical Case. Toxins, 2(11), 2584-2592. DOI
- Miura, M., Tada, H., Yasui-Furukori, N., Uno, T., Sugawara, K., Tateishi, T., Suzuki, T. (2004) Pharmacokinetic differences between the enantiomers of lansoprazole and its metabolite, 5-hydroxylansoprazole, in relation to CYP2C19 genotypes. Eur. J. Clin. Pharmacol., 60, 623-628. DOI
- Wienkers, L.C., Wurden, C.J., Storch, E., Kunz,e K.L., Rettie, A.E., Trager, W.F. (1996) Formation of (R)-8-hydroxywarfarin in human liver microsomes. A new metabolic marker for the (S)-mephenytoin hydroxylase, P4502C19. Drug Metab. Dispos., 24(5), 610-614. DOI
- Klaassen, T., Jetter, A., Tomalik-Scharte, D., Kasel, D., Kirchheiner, J., Jaehde, U., Fuhr, U. (2008) Assessment of urinary mephenytoin metrics to phenotype for CYP2C19 and CYP2B6 activity. Eur. J. Clin. Pharmacol., 64, 387-398. DOI
- Ebner, T., Eichelbaum, M. (1993) The metabolism of aprindine in relation to the sparteine/debrisoquine polymorphism. British Journal of Clinical Pharmacology, 35, 426-430. DOI
- Dinh, J.C., Pearce, R.E., Van Haandel, L., Gaedigk, A., Leeder, J.S. (2016) Characterization of atomoxetine biotransformation. Drug Metabolism and Disposition, 44(7), 1070-1079. DOI
- Nguyen, H.Q., Callegari, E., Obach, R.S. (2016) The use of in vitro data and physiologically-based pharmacokinetic modeling to predict drug metabolite exposure: desipramine exposure in cytochrome P4502D6 extensive and poor metabolizers following administration of imipramine. Drug Metab. Dispos., 44(10), 1569-1578. DOI
- Lantz, R.J., Gillespie, T.A., Rash, T.J., Kuo, F., Skinner, M., Kuan, H-Y., Knadler, M.P. (2003) Metabolism, excretion, and pharmacokinetics of duloxetine in healthy human subjects. Drug Metabolism and Disposition, 31(9), 1142-1150. DOI
- Wang, Z., Wang, L., Xu, R., Zhan, Y., Huang, C., Dai, D., Cai, J., Hu ,G. (2016) Role of cytochrome P450 2D6 genetic polymorphism in carvedilol hydroxylation in vitro. Drug Design, Development and Therapy, 10, 1909-1916. DOI
- Bakken, G.V., Rudberg, I., Christensen, H., Molden, E., Refsum, H., Hermann, M. (2009) Metabolism of quetiapine by CYP3A4 and CYP3A5 in presence or absence of cytochrome B5. Drug Metabolism and Disposition, 37(2), 254-258. DOI
- Funae, Y., Kishimoto, W., Cho, T., Niwa, T., Hiroi, T. (2003) CYP2D in the brain. Drug metabolism and pharmacokinetics, 18(6), 337-349. DOI
- Okubo, M., Murayama, N., Miura, J., Chiba, Y., Yamazaki, H. (2015) Effects of cytochrome P450 2D6 and 3A5 genotypes and possible coadministered medicines on the metabolic clearance of antidepressant mirtazapine in Japanese patients. Biochemical Pharmacology, 93(1), 104-109. DOI
- Katsunori, N., Tsuyoshi, Y., Kazuaki, I., Noriaki, S., Noriko, O., Toshiyuki, K., Tetsuya, K. (1996) CYP2D6 is the principal cytochrome P450 responsible for metabolism of the histamine 111 antagonist promethazine in human liver microsomes. Pharmacogenetics, 6(5), 449-457. DOI
- Afshar, M., Rouini, M. (2004) A Rapid HPLC Assay for the simultaneous determination of propafenone and its major metabolites in human serum. Analytical Sciences, 20(9), 1307-1311. DOI
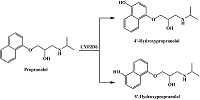
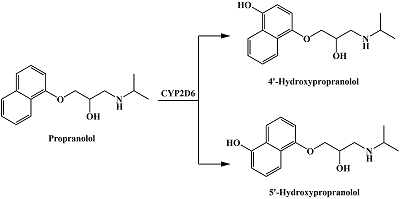
- Upthagrove, A.L., Nelson, W.L. (2001) Importance of amine pKa and distribution coefficient in the metabolism of fluorinated propranolol derivatives. Preparation, identification of metabolite regioisomers, and metabolism by CYP2D6. Drug Metab. Dispos., 29(11), 1377-1388.
- Lyon, E., Gastier Foster, J., Palomaki, G.E., Pratt, V.M., Reynolds, K., Sábato, M.F., Scott S.A., Vitazka P. (2012) Working group of the Molecular Genetics Subcommittee on behalf of the American College of Medical Genetics and Genomics (ACMG) Laboratory Quality Assurance Committee. Laboratory testing of CYP2D6 alleles in relation to tamoxifen therapy. Genet. Med., 14(12), 990-1000. DOI
- Beloti, L.G.M., Miranda, L.F.C., Queiroz, M.E.C. (2019) Butyl methacrylate-co-ethylene glycol dimethacrylate monolith for online in-tube SPME-UHPLC-MS/MS to Determine chlopromazine, clozapine, quetiapine, olanzapine, and their metabolites in plasma samples. Molecules, 24(2), 310. DOI
- Mürdter, T.E., Kerb, R., Turpeinen, M., Schroth, W., Ganchev, B., Böhmer, G.M., Igel, S., Schaeffeler, E., Zanger, U., Brauch ,H., Schwab, M. (2012) Genetic polymorphism of cytochrome P450 2D6 determines oestrogen receptor activity of the major infertility drug clomiphene via its active metabolites. Human Molecular Genetics, 21(5), 1145-1154. DOI
- Yamamura, Y., Koyama, N., Umehara, K. (2015) Comprehensive kinetic analysis and influence of reaction components for chlorzoxazone 6-hydroxylation in human liver microsomes with CYP antibodies. Xenobiotica, 45(4), 353-360. DOI
- Liu, A., Wu, Q., Guo, J., Ares, I., Rodríguez, J.-L., Martínez-Larrañaga, M.-R., Yuan, Z., Anadón, A., Wang, X., Martínez, M.-A. (2019) Statins: adverse reactions, oxidative stress and metabolic interactions. Pharmacology & Therapeutics, 195, 54-84. DOI
- Masubuchi, Y., Ose, A., Horie, T. (2002) Diclofenac-induced inactivation of CYP3A4 and its stimulation by quinidine. Drug Metab. Dispos., 30(10), 1143-1148. DOI
- Fan-Havard, P., Liu, Z., Chou, M., Ling, Y., Barrail-Tran, A., Haas, D.W., Taburet, A.M., ANRS12154 Study Group (2013) Pharmacokinetics of phase I nevirapine metabolites following a single dose and at steady state. Antimicrob. Agents Chemother., 57(5), 2154-2160. DOI
- Wen, B., Ma, L., Rodrigues, A.D., Zhu, M. (2008) Detection of novel reactive metabolites of trazodone: evidence for CYP2D6-mediated bioactivation of m-chlorophenylpiperazine. Drug Metabolism and Disposition, 36(5), 841-850. DOI
- Zhang, H., Cui, D., Wang, B., Han, Y.-H., Balimane, P., Yang, Z., Sinz, M., Rodrigues, A.D. (2007) Pharmacokinetic drug interactions involving 17α-ethinylestradiol. Clin. Pharmacokinet., 46, 133-157. DOI
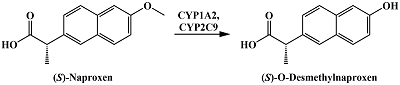
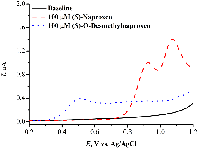
- Miners, J.O., Coulter, S., Tukey, R.H., Veronese, M.E., Birkett ,D.J. (1996) Cytochromes P450, 1A2, and 2C9 are responsible for the human hepatic O-demethylation of R- and S-naproxen. Biochemical Pharmacology, 51(8), 1003-1008. DOI
- Taxak, N., Prasad, K., Bharatam, P. (2012) Mechanistic insights into the bioactivation of phenacetin to reactive metabolites: A DFT study. Comput. Theor. Chem., 1007, 48-56. DOI
- Nakajima, M., Inoue, T., Shimada, N., Tokudome, S., Yamamoto, T., Kuroiwa, Y. (1998) Cytochrome P450 2C9 catalyzes indomethacin O-demethylation in human liver microsomes. Drug Metab. Dispos., 26(3), 261-266.
- Pan, P.P., Weng, Q.H., Zhou, C.J., Wei, Y.L., Wang, L., Dai, D.P., Cai, J.P., Hu, G.X. (2016) The role of CYP2C9 genetic polymorphism in carvedilol O-desmethylation in vitro. Eur. J. Drug Metab. Pharmacokinet., 41(1), 79-86. DOI
- Magalhães, P., Alves, G., Llerena, A., Falcão, A. (2014) Venlafaxine pharmacokinetics focused on drug metabolism and potential biomarkers. Drug Metabol. Drug Interact., 29(3), 129-141. DOI
- Bachus, R., Bickel, U., Thomsen, T., Roots, I., Kewitz, H. (1999) The O-demethylation of the antidementia drug galanthamine is catalysed by cytochrome P450 2D6. Pharmacogenetics, 9(6), 661-668. DOI
- Fang, P., Zheng, X., He, J., Ge, H., Tang, P., Cai, J., Hu, G. (2017) Functional characterization of wild-type and 24 CYP2D6 allelic variants on gefitinib metabolism in vitro. Drug Des. Devel. Ther., 11, 1283-1290. DOI
- Barakat, N.H., Atayee, R.S., Best, B.M., Pesce, A.J. (2012) Relationship between the concentration of hydrocodone and its conversion to hydromorphone in chronic pain patients using urinary excretion data. J. Anal. Toxicol., 36(4), 257-264. DOI
- Kim, J., Lim, Y.R., Han, S., Han, J.S., Chun, Y.J., Yun, C.H., Lee, C.H., Kim, D. (2013) Functional influence of human CYP2D6 allelic variations: P34S, E418K, S486T, and R296C. Arch. Pharm. Res., 36(12), 1500-1506. DOI
- Mikus, G., Weiss, J. (2005) Influence of CYP2D6 genetics on opioid kinetics, metabolism and response. Current Pharmacogenomics, 3(1), 43-52. DOI
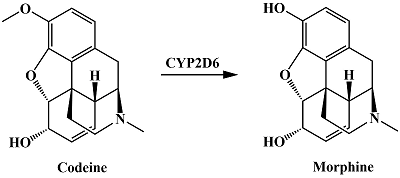
- Shord, S.S., Cavallari, L.H., Gao, W., Jeong, H.Y., Deyo, K., Patel, S.R., Camp, J.R., Labott, S.M., Molokie ,R.E. (2009) The pharmacokinetics of codeine and its metabolites in Blacks with sickle cell disease. Eur. J. Clin. Pharmacol., 65(7), 651-658. DOI
- Samer, C.F., Daali, Y., Wagner, M., Hopfgartner, G., Eap, C.B., Rebsamen, M.C., Rossier, M.F., Hochstrasser, D., Dayer, P., Desmeules, J.A. (2010) Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety. Br. J. Pharmacol., 160(4), 919-930. DOI
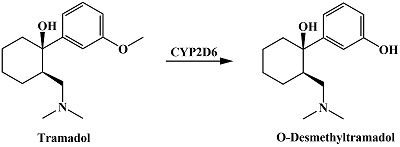
- Perez Jimenez, T.E., Mealey, K.L., Grubb, T.L., Greene, S.A., Court, M.H. (2016) Tramadol metabolism to O-desmethyl tramadol (M1) and N-desmethyl tramadol (M2) by dog liver microsomes: Species comparison and identification of responsible canine cytochrome P-450s (CYPs). Drug Metab. Dispos., 44(12), 1963-1972. DOI
- Doki, K., Sekiguchi, Y., Kuga, K., Aonuma, K., Homma, M. (2015) Serum flecainide S/R ratio reflects the CYP2D6 genotype and changes in CYP2D6 activity. Drug Metab. Pharmacokinet., 30(4), 257-262. DOI
- Matsumoto, S., Hirama, T., Matsubara, T., Nagata, K., Yamazoe, Y. (2002) Involvement of CYP2J2 on the intestinal first-pass metabolism of antihistamine drug, astemizole. Drug Metab. Dispos., 30(11), 1240-1245. DOI
- Wilkinson, D.G. (1999) The pharmacology of donepezil: a new treatment of Alzheimer's disease. Expert Opin. Pharmacother., 1(1), 121-135. DOI
- Zanger, U.M., Schwab, M. (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther., 138(1), 103-141. DOI
- Jurva, U., Wikström, H.V., Weidolf, L., Bruins, A.P. (2003) Comparison between electrochemistry/mass spectrometry and cytochrome P450 catalyzed oxidation reactions. Rapid Commun. Mass Spectrom., 17(8), 800-810. DOI
- Nouri-Nigjeh, E., Bischoff, R., Bruins, A.P., Permentier, H.P. (2011) Electrochemistry in the mimicry of oxidative drug metabolism by cytochrome P450s. Curr. Drug Metab., 12(4), 359-371. DOI
- Kaminsky, L.S., Zhang, Z.Y. (1997) Human P450 metabolism of warfarin. Pharmacol. Ther., 73(1), 67-74. DOI
- Suckow, R.F., Cooper, T.B., Quitkin, F.M., Stewart, J.W. (1982) Determination of mianserin and metabolites in plasma by liquid chromatography with electrochemical detection. J. Pharm. Sci., 71(8), 889-892. DOI
- Koyama, E., Kikuchi, Y., Echizen, H., Chiba, K., Ishizaki, T. (1993) Simultaneous high-performance liquid chromatography-electrochemical detection determination of imipramine, desipramine, their 2-hydroxylated metabolites, and imipramine N-oxide in human plasma and urine: preliminary application to oxidation pharmacogenetics. Ther. Drug Monit., 15(3), 224-235. DOI
- Bogni, A., Monshouwer, M., Moscone, A., Hidestrand, M., Ingelman-Sundberg, M., Hartung, T., Coecke, S. (2005) Substrate specific metabolism by polymorphic cytochrome P450 2D6 alleles. Toxicol. In Vitro, 19(5), 621-629. DOI
- DeVane, C.L. (2006) Antidepressant-drug interactions are potentially but rarely clinically significant. Neuropsychopharmacology, 31(8), 1594-1604; discussion 1614-1615. DOI
- Tang, W. (2003) The metabolism of diclofenac - enzymology and toxicology perspectives. Curr. Drug Metab., 4(4), 319-329. DOI
- Goyal, R.N., Chatterjee, S., Agrawal, B. (2010) Electrochemical investigations of diclofenac at edge plane pyrolytic graphite electrode and its determination in human urine. Sensors and Actuators B: Chemical, 145(2), 743-748. DOI
- Cid-Cerón, M.M., Guzmán-Hernández, D.S., Ramírez-Silva, M.T., Galano, A., Romero-Romo, M., Palomar-Pardavé, M. (2016) New insigths on the kinetics and mechanism of the electrochemical oxidation of diclofenac in neutral aqueous medium. Electrochimica Acta, 199, 92-98. DOI
- Aguilar-Lira, G.Y., Álvarez-Romero, G.A., Zamora-Suárez, A., Palomar-Pardavé, M., Rojas-Hernández, A., Rodríguez-Ávila, J.A., Páez-Hernández, M.E. (2017) New insights on diclofenac electrochemistry using graphite as working electrode. Journal of Electroanalytical Chemistry, 794, 182-188. DOI
- Madsen, K.G., Skonberg, C., Jurva, U., Cornett, C., Hansen, S.H., Johansen, T.N., Olsen, J. (2008) Bioactivation of diclofenac in vitro and in vivo: correlation to electrochemical studies. Chem. Res. Toxicol., 21(5), 1107-1119. DOI
- Zi, J., Liu, D., Ma, P., Huang, H., Zhu, J., Wei, D., Yang, J., Chen, C. (2010) Effects of CYP2C9*3 and CYP2C9*13 on Diclofenac metabolism and inhibition-based drug-drug interactions. Drug Metab. Pharmacokinet., 25(4), 343-350. DOI
- Suwa, T., Urano, H., Shinohara, Y., Kokatsu, J. (1993) Simultaneous high-performance liquid chromatographic determination of lornoxicam and its 5'-hydroxy metabolite in human plasma using electrochemical detection. J. Chromatogr., 617(1), 105-110. DOI
- McCreery, R.L. (1977) Oxidative reactions of hydroxylated chlorpromazine metabolites. J. Pharm. Sci., 66(3), 357-361. DOI
- Neptune, M., McCreery, R.L. (1978) Chemical and electrochemical oxidation of 7-hydroxychlorpromazine. J. Med. Chem., 21(4), 362-368. DOI
- Baranowska, I., Koper, M. (2011) Electrochemical behavior of Propranolol and its major metabolites, 4'-hydroxypropranolol and 4'-hydroxypropranolol Sulfate, on glassy carbon electrode. J. Braz. Chem. Soc., 22(8), 1601-1609. DOI
- Santos, S.X.d., Cavalheiro, É.T.G., Brett, C.M.A. (2010) Analytical potentialities of carbon nanotube/silicone rubber composite electrodes: determination of propranolol. Electroanalysis, 22, 2776-2783. DOI
- Santos, S.X.d., Cavalheiro, É.T.G., Brett, C.M.A. (2011) The potentialities of using a graphite-silicone rubber composite electrode in the determination of propranolol. Analytical Letters, 44(5), 850-862. DOI
- Stoian, I.-A., Iacob, B.-C., Ramalho, J.P.P., Marian, I.O., Chiș, V., Bodoki, E., Oprean, R. (2019) A chiral electrochemical system based on l-cysteine modified gold nanoparticles for propranolol enantiodiscrimination: Electroanalysis and computational modelling. Electrochimica Acta, 326, 134961. DOI
- Yin, H., Meng, X., Xu, Z., Chena, L., Ai S. (2012) Electrochemical behavior of phenacetin on CdSe microspheres modified glassy carbon electrode and its simultaneous determination with paracetamol and 4-aminophenol. Analytical Methods, 4, 1445-1451. DOI
- Smith, H.S. (2009) Opioid metabolism. Mayo Clin. Proc., 84(7), 613-624. DOI
- Wester, N., Mynttinen, E., Etula, J., Lilius, T., Kalso, E., Kauppinen, E.I., Laurila, T., Koskinen, J. (2019) Simultaneous detection of morphine and codeine in the presence of ascorbic acid and uric acid and in human plasma at nafion single-walled carbon nanotube Thin-Film Electrode. ACS Omega, 4(18), 17726-17734. DOI
- Mynttinen, E., Wester, N., Lilius, T., Kalso, E., Koskinen, J., Laurila, T. (2019) Simultaneous electrochemical detection of tramadol and O-desmethyltramadol with Nafion-coated tetrahedral amorphous carbon electrode. Electrochimica Acta, 295, 347-353. DOI
- Sadeghi, S.J., Fantuzzi, A., Gilardi, G. (2011) Breakthrough in P450 bioelectrochemistry and future perspectives. Biochim. Biophys. Acta, 1814(1), 237-248. DOI
- Schneider, E., Clark, D.S. (2013) Cytochrome P450 (CYP) enzymes and the development of CYP biosensors. Biosens. Bioelectron., 39(1), 1-13. DOI
- Shumyantseva, V.V., Kuzikov, A.V., Masamrekh, R.A., Bulko, T.V., Archakov, A.I. (2018) From electrochemistry to enzyme kinetics of cytochrome P450. Biosens. Bioelectron., 121, 192-204. DOI
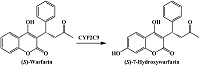
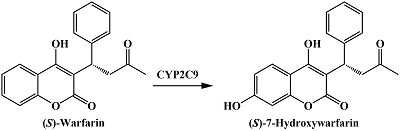
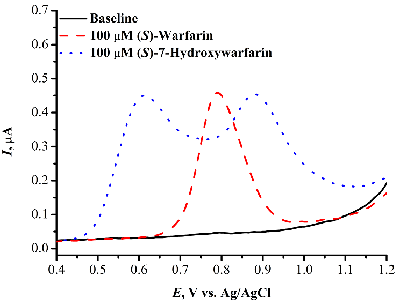
- Kuzikov, A.V., Filippova, T.A., Masamrekh, R.A., Shumyantseva, V.V. (2022) Electrochemical determination of (S)-7-hydroxywarfarin for analysis of CYP2C9 catalytic activity. Journal of Electroanalytical Chemistry, 904, 115937. DOI
- Mosher, C.M., Hummel, M.A., Tracy, T.S., Rettie, A.E. (2008) Functional analysis of phenylalanine residues in the active site of cytochrome P450 2C9. Biochemistry, 47(45), 11725-11734. DOI
- Michalkiewicz, S., Skorupa, A., Jakubczyk, M. (2021) Carbon Materials in Electroanalysis of Preservatives: A Review. Materials (Basel), 14(24), 7630. DOI
- Kuzikov, A.V., Filippova, T.A., Masamrekh, R.A., Shumyantseva, V.V. (2022) Electroanalysis of 4′-Hydroxydiclofenac for CYP2C9 Enzymatic Assay. Electrocatalysis, 13, 630-640. DOI
- Haidukevich, I.V., Sushko, T.A., Tumilovich, A.M., Grabovec, I.P., Usanov, S.A., Gilep, A.A. (2018) Different inhibitory effects of azole-containing drugs and pesticides on CYP2C9 polymorphic forms: An in vitro study. Toxicology in Vitro, 50, 249-256. DOI
- Sohl, C.D., Guengerich, F.P. (2010) Kinetic analysis of the three-step steroid aromatase reaction of human cytochrome P450 19A1. J. Biol. Chem., 285(23), 17734-17743. DOI
- Zhu, Y., Liu, X., Jia, J. (2015) Electrochemical detection of natural estrogens using a graphene/ordered mesoporous carbon modified carbon paste electrode. Analytical Methods, 7, 8626-8631. DOI
- Moraes, F.C., Rossi, B., Donatoni, M.C., de Oliveira K.T., Pereira E.C. (2015) Sensitive determination of 17β-estradiol in river water using a graphene based electrochemical sensor. Anal. Chim. Acta, 881, 37-43. DOI
- Lin, X., Li, Y. (2006) A sensitive determination of estrogens with a Pt nano-clusters/multi-walled carbon nanotubes modified glassy carbon electrode. Biosens. Bioelectron., 22(2), 253-259. DOI
- Hu, S., Wu, K., Yi, H., Cui, D. (2002) Voltammetric behavior and determination of estrogens at Nafion-modified glassy carbon electrode in the presence of cetyltrimethylammonium bromide. Analytica Chimica Acta, 464(2), 209-216. DOI
- Musa, A.M., Kiely, J., Luxton, R., Honeychurch, K.C. (2021) Recent progress in screen-printed electrochemical sensors and biosensors for the detection of estrogens. TrAC Trends in Analytical Chemistry, 139, 116254. DOI
- Kuzikov, A.V., Masamrekh, R.A., Filippova, T.A., Haurychenka, Y.I., Gilep, A.A., Shkel, T.V., Strushkevich, N.V., Usanov, S.A., Shumyantseva, V.V. (2020) Electrochemical oxidation of estrogens as a method for CYP19A1 (aromatase) electrocatalytic activity determination. Electrochimica Acta, 333, 135539. DOI
Supplementary materials are available at http://dx.doi.org/10.18097/BMCRM00174
REFERENCES