Selection of the Most Efficient Protocol for the Immunoglobulin Y Extraction from Hen Egg Yolk
Institute of Biomedical Chemistry, Pogodinskaya Street, 10, Moscow 119121, Russia; *e-mail: ekaterina.kolesanova@ibmc.msk.ru
Keywords: immunoglobulins Y, extraction and purification, hen egg yolk, delipidation, sodium sulfate precipitation, PAAG electrophoresis
DOI:10.18097/BMCRM00179
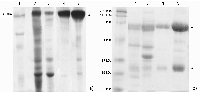
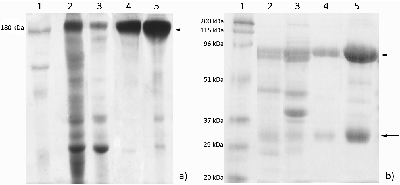
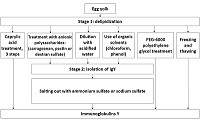
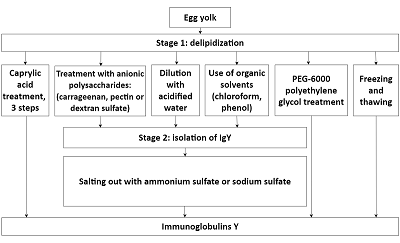
Four protocols of immunoglobulin Y extraction and purification from hen egg yolk were compared and the optimal one was chosen from the viewpoint of the purity and yield of the final protein preparation. The following protocols were tested: 1) three-step treatment of the yolk substance with caprylic acid; 2) delipidation with dextran-sulfate followed by sodium sulfate fractionation; 3) removal of lipids via diluting by acidified water followed by sodium sulfate fractionation and 4) purification of immunoglobulins with the use of egg yolk freezing-thawing. Protein yields were assessed as amounts of the total protein in the final immunoglobulin preparations; purity was assessed via polyacrylamide gel electrophoresis in denaturing (reducing and non-reducing) conditions. The protocol of the immunoglobulin Y extraction with the removal of lipids via diluting by acidified water followed by sodium sulfate fractionation was considered as the optimal one, with regard to the ratio between the protein yield and immunoglobulin preparation purity. This protocol can be employed both for the preparation of immunoglobulin Y samples for further affinity purifications of specific antibodies for research purposes and for the production of immunoglobulins Y as pharmaceutics.
|
CLOSE

|
Table 1.
Characteristics of IgY preparations produced via different protocols.
|
FUNDING
The work was performed within the framework of the Program for Basic Research in the Russian Federation for a long-term period (2021-2030), theme No.122030100170-5.
REFERENCES
- Mine, A., Kovacs-Nolan, J. (2002) Chicken egg yolk antibodies as therapeutics in enteric infectious disease: a review. Journal of Medicinal Food, 5(3), 159–169. DOI
- Larsson, A., Karlsson-Parra, A., & Sjöquist, J. (1991) Use of chicken antibodies in enzyme immunoassays to avoid interference by rheumatoid factors. Clinical Chemistry, 37(3), 411–414. DOI
- Larsson, A., Wejåker, P. E., Forsberg, P.O., Lindahl, T. (1992) Chicken antibodies: a tool to avoid interference by complement activation in ELISA. Journal of Immunological Methods, 156(1), 79–83. DOI
- Bocharova, O.V., Moshkovskij, S.A., Chertkova, R.V., Abdullaev, Z.H., Kolesanova, E.F., Dolgih, D.A., Kirpichnikov, M.P. (2002) Vvedenie biologicheski aktivnyh fragmentov interferona-a2 i insulina v sostav iskusstvennogo belka al'bebetina vliyaet na immunogennost' itogovoj konstrukcii. Voprosy Meditsinskoj Himii, 48(1), 94-102.
- Moshkovskii, S.A., Kolesanova, E.F., Archakov, A.I. (2002) Continuous B-epitope maps of cytochrome P450cam (CYP101) obtained by peptide scanning: correlation to spatial structure. Archives of Biochemistry and Biophysics, 398, 269-274. DOI
- Lu, Y., Wang, Y., Zhang, Z., Huang, J., Yao, M., Huang, G., Ge, Y., Zhang, P., Huang, H., Wang, Y., Li, H., Wang, W. (2020) Generation of Chicken IgY against SARS-COV-2 Spike Protein and Epitope Mapping. Journal of Immunology Research, 2020, 9465398. DOI
- Somasundaram, R., Choraria, A., Antonysamy, M. (2020) An approach towards development of monoclonal IgY antibodies against SARS CoV-2 spike protein (S) using phage display method: A review. International Immunopharmacology, 85, 106654. DOI
- Palaniyappan, A., Das, D., Kammila, S., Suresh, M. R., Sunwoo, H. H. (2012) Diagnostics of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid antigen using chicken immunoglobulin Y. Poultry Science, 91(3), 636-642. DOI
- Su Y., Sun Y., Zhai Y., Gu L., Li J., Gong L., Chang C., Yang Y. Effects of surfactants on activity and structure of egg yolk antibody. Food and Bioproducts Processing, 132, 167-176. DOI
- Redwan, E.M., Aljadawi, A.A., Uversky, V.N. (2021) Simple and efficient protocol for immunoglobulin Y purification from chicken egg yolk. Poultry Science, 100(3), 100956. DOI
- Jensenius, J.C., Andersen, I., Hau, J., Crone, M., Koch, C. (1981) Eggs: conveniently packaged antibodies. Methods for purification of yolk IgG. Journal of Immunological Methods, 46(1), 63-68. DOI
- Akita, E.M., Nakai, S. (1992) Immunoglobulins from Egg Yolk: Isolation and Purification. Journal of Food Science, 57, 629-634. DOI
- Akita, E.M., Nakai, S. (1993) Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic E. coli strain. Journal of Immunological Methods, 160(2), 207-214. DOI
- Wang, X., Li, J., Su, Y., Chang, C., Yang, Y. (2021) Freeze-thaw treatment assists isolation of IgY from chicken egg yolk. Food Chemistry, 364, 130225. DOI
- Polson, A., Coetzer, T., Kruger, J., von Maltzahn, E., van der Merwe, K. J. (1985) Improvements in the isolation of IgY from the yolks of eggs laid by immunized hens. Immunological investigations, 14(4), 323-327. DOI
- Fishman, J. B., Berg, E. A. (2018) Isolation of IgY from Chicken Eggs. Cold Spring Harbor Protocols, 2018(6). DOI
- Ren, H., Yang, W., Thirumalai, D., Zhang, X., & Schade, R. (2016) A comparative evaluation of six principal IgY antibody extraction methods. Alternatives to Laboratory Animals : ATLA, 44(1), 11-20. DOI
- Chang, H. M., Lu, T. C., Chen, C. C., Tu, Y. Y., Hwang, J. Y. (2000) Isolation of immunoglobulin from egg yolk by anionic polysaccharides. Journal of Agricultural and Food Chemistry, 48(4), 995-999. DOI
- Gassmann, M., Thömmes, P., Weiser, T., Hübscher, U. (1990) Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. FASEB journal, 4(8), 2528-2532. DOI
- Pereira, E., van Tilburg, M. F., Florean, E., Guedes, M. (2019) Egg yolk antibodies (IgY) and their applications in human and veterinary health: A review. International Immunopharmacology, 73, 293–303. DOI
- Rajesvari, S., Choraria, A., Xiao-Ying Zhang, X.-Y., Antonysamy, M. (2018) Applications of Chicken Egg Yolk Antibodies (IgY) in healthcare: A review. Biomedical Journal of Scientific and Technology Research 2(1), 2161-2163. DOI
- Abbas, A. T., El-Kafrawy, S. A., Sohrab, S. S., Azhar, E. (2019) IgY antibodies for the immunoprophylaxis and therapy of respiratory infections. Human Vaccines & Immunotherapeutics, 15(1), 264-275. DOI
- Constantin, C., Neagu, M., Diana Supeanu, T., Chiurciu, V., & A Spandidos, D. (2020) IgY - turning the page toward passive immunization in COVID-19 infection (Review). Experimental and Therapeutic Medicine, 20(1), 151–158. DOI
- Lyu, J., Bao, L., Shen, X., Yan, C., Zhang, C., Wei, W., Yang, Y., Li, J., Dong, J., Xiao, L., Zhou, X., Li, Y. (2021) The preparation of N-IgY targeting SARS-CoV-2 and its immunomodulation to IFN-γ production in vitro. International Immunopharmacology, 96, 107797. DOI
- Aston, E.J., Wallach, M.G., Narayanan, A., Egaña-Labrin, S., Gallardo, R.A. (2022) Hyperimmunized chickens produce neutralizing antibodies against SARS-CoV-2. Viruses 14(7):1510. DOI
- Agurto-Arteaga, A., Poma-Acevedo, A., Rios-Matos, D., Choque-Guevara, R., Montesinos-Millán, R., Montalván, Á., Isasi-Rivas, G., Cauna-Orocollo, Y., Cauti-Mendoza, M. G., Pérez-Martínez, N., Gutierrez-Manchay, K., Ramirez-Ortiz, I., Núñez-Fernández, D., Salguedo-Bohorquez, M. I., Quiñones-Garcia, S., Fernández Díaz, M., Guevara Sarmiento, L. A., Zimic, M., COVID-19 Working Group in Perú. (2022) Preclinical Assessment of IgY Antibodies Against Recombinant SARS-CoV-2 RBD Protein for Prophylaxis and Post-Infection Treatment of COVID-19. Frontiers in Immunology, 13, 881604. DOI
- Wallach, M. G. (2022). Opinion: The use of chicken IgY in the control of pandemics. Frontiers in Immunology, 13, 954310. DOI
- Laemmli, U.K. (1970) Cleavage of structural proteins during the assemblyof the head of bacteriophage T4. Nature; 227, 680-685. DOI
- Generalov, I. I., Moiseeva, A. M., Zherulik, S. V., Generalova, A. G., & Zheleznyak, N. V. (2009) Poluchenie vy`sokoochishhenny`x immunoglobulinov klassa g iz sy`vorotki krovi cheloveka. Vestnik Farmacii, 4(46), 52-60.
- GelAnalyzer 19.1 by I. Lazar, Jr., and I. Lazar, Sr., URL: www.gelanalyzer.com