Influence of Derivatization Temperature and Butylated Hydroxytoluene on the Content of Malondialdehyde in Liver Homogenates
1Federal Research Centre of Nutrition, Biotechnology and Food Safety,
2/14 Ustinsky pas., Moscow, 109240 Russia; *e-mail: konevtonyion@gmail.com
2I.M. Sechenov First Moscow State Medical University, 8 Trubetskaya str., Moscow, 119048 Russia
Keywords: lipid peroxidation; malondialdehyde; derivatization temperature; butylated hydroxytoluene; 2-thiobarbituric acid; liver homogenate.
DOI:10.18097/BMCRM00215
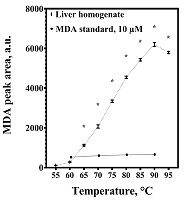
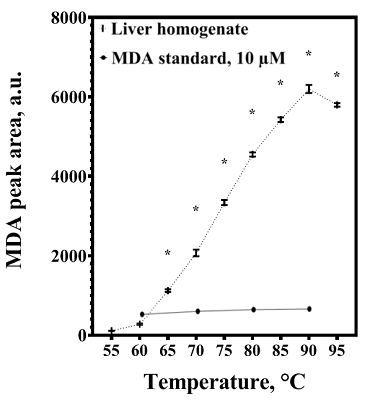
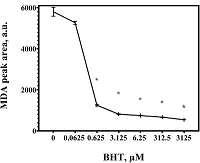
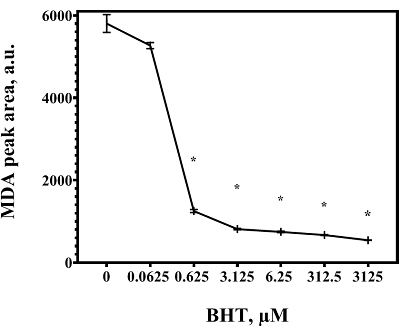
Malondialdehyde (MDA) is a product of lipid peroxidation that is widely used as a marker of oxidative stress in biomedical research. Detectable levels of MDA can vary significantly, which may be due to its formation in vitro during sample preparation. The purpose of the work was to analyze the methodological reasons for overestimating the malondialdehyde content in the liver and to find approaches to eliminate the flaws of the method. The amount of MDA was estimated by its derivatization with 2-thiobarbituric acid (TBA) with subsequent analysis by HPLC with fluorimetric detection. Increasing the derivatization temperature had no significant effect on the intensity of MDA-TBA complex formation when standard MDA solutions were used, but led to a sharp increase in its content in liver homogenates, which was dose-dependently prevented by the inclusion of butylhydroxytoluene (BHT) in the reaction mixture. The results obtained may be in demand for the development of methods for the analysis of MDA in organs and tissues, as well as for the interpretation of relevant data from biomedical studies.
FUNDING
This work was carried out using subsidies for the implementation of a state task in the Russian Federation (research No. FGMF-2023-0006).
REFERENCES
- Janero, D.R. (1990) Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med., 9(6), 515-540. DOI
- Del Rio, D., Stewart, A.J., Pellegrini, N. (2005) A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis., 15(4), 316-328. DOI
- Tsikas, D. (2017) Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem., 524, 13-30. DOI
- Romuk, E.B., Szczurek, W., Nowak, P.G., Hudziec, E., Chwalinska, E., Birkner, E. (2016) Effects of propofol on the liver oxidative-antioxidant balance in a rat model of Parkinson's disease. Adv. Clin. Exp. Med., 25(5), 815-820. DOI
- Ohkawa, H., Ohishi, N., Yagi, K. (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem., 95(2), 351-358. DOI
- Koyu, A., Gokcimen, A., Ozguner, F., Bayram, D.S., Kocak, A. (2006) Evaluation of the effects of cadmium on rat liver. Mol. Cell. Biochem., 284, 81-85. DOI
- Draper, H.H., Hadley, M. (1990) Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol., 186, 421-431. DOI
- Domijan, A.M., Ralic, J., Brkanac, S. R., Rumora, L., Zanic-Grubisie, T. (2015) Quantification of malondialdehyde by HPLC-FL - application to various biological samples. Biomed. Chromatogr., 29(1), 41-46. DOI
- Pikul, J., Leszczynski, D.E., Kummerow, F.A. (1983) Elimination of sample autoxidation by butylated hydroxytoluene additions before thiobarbituric acid assay for malonaldehyde in fat from chicken meat. Journal of Agricultural and Food Chemistry, 31(6), 1338-1342. DOI
- Pikul, J., Leszczynski, D.E. (1986) Butylated hydroxytoluene addition improves the thiobarbituric acid assay for malonaldehyde from chicken plasma fat. Nahrung, 30(7), 673-678. DOI
- Biochem. Toxicol.: A Practical Approach, Lake B.G. 1987, Oxford University Press: Oxford. p. 183-212.
- Reeves, P.G., Nielsen, F.H., Fahey Jr., G.C. (1993) AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr., 123(11),1939-1951. DOI
- Repetto, M. G., Ferrarotti, N. F., Boveris, A. (2009) The involvement of transition metal ions on iron-dependent lipid peroxidation. Archives of Toxicology, 84(4), 255-262. DOI
- Miller, D.M., Buettner, G.R., Aust, S.D. (1990) Transition metals as catalysts of "autoxidation" reactions. Free Radic. Biol. Med., 8(1), 95-108. DOI
- Vladimirov, Y.A., Archakov, A.I. (1972) Perekisnoe okislenie lipidov v biolohicheskix membranah, Nauka: Moskow. p. 52-95.
- Tuma, D.J. (2002) Role of malondialdehyde-acetaldehyde adducts in liver injury. Free Radic. Biol. Med., 32(4), 303-308. DOI
- Niemelä, O., Parkkila, S., Ylä-Herttuala, S., Villanueva, J., Ruebner, B., Halsted, C.H. (1995) Sequential acetaldehyde production, lipid peroxidation, and fibrogenesis in micropig model of alcohol-induced liver disease. Hepatology, 22(4), 1208-1214. DOI
- Chen, J., Petersen, D.R., Schenker, S., Henderson, G.I. (2000) Formation of malondialdehyde adducts in livers of rats exposed to ethanol: role in ethanol-mediated inhibition of cytochrome c oxidase. Alcohol Clin. Exp. Res., 24(4), 544-552. DOI