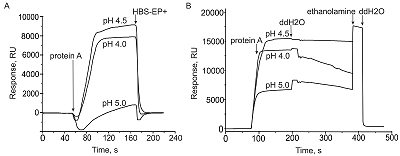
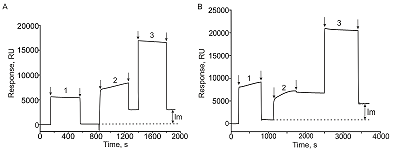
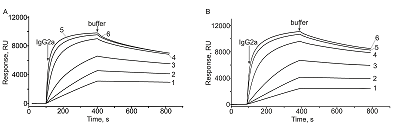
Comparative SPR Analysis of Intermolecular Interactions Performed Using the Original Biacore CM5 CHIP and its Analog CMD500M
Institute of Biomedical Chemistry, Pogodinskaya str., 10, Moscow 119121, Russia; *e-mail: gnedenko.oksana@gmail.com
Keywords:surface plasmon resonance (SPR); carboxymethylated dextran; antigen-antibody interaction; kinetic constants (kon, koff); equilibrium dissociation constant (Kd); CM5 chips and their analogues CMD500M
DOI:10.18097/BMCRM00220
Currently, users of Biacore SPR biosensors (“Cytiva”, USA) are faced with sanctions restrictions on the purchase of consumables (primarily optical chips) for this type of equipments. In this regard, the use of commercially available analogues of the optical chips has become relevant. In this work, a comparative study of molecular interactions was performed on a Biacore X100 SPR biosensor using an original Biacore CM5 optical chip (“Cytiva”, USA) and its analogue CMD500M (“XanTec bioanalytics GmbH”, Germany). Protein A was immobilized on both chips as a molecular ligand, often used in scientific research and biotechnological works to immobilize antibodies on various carriers (biosensor chips, sorbents, nano- and microparticles). An IgG antibody was used as a protein analyte. A comparative study of the interaction of various concentrations of antibodies with protein A immobilized on two versions of the chips was carried out. The values of the kinetic rate constants for the association (kon) and dissoсiation (koff) of complexes, as well as the equilibrium dissociation constant (Kd), were calculated from the obtained sensorgrams using the interaction model 1:1 (Langmuir) binding. The results of comparative measurements showed similar values of the rate constants and interaction affinities. The differences between the values of kon, koff and Kd were 18%, 10% and 9%, respectively. Thus, this study confirmed the interchangeability of the original SPR chips CM5 and their analogues CMD500M.
|
CLOSE

|
Table 1.
Protein-ligand (protein A) immobilization protocols performed in accordance with the manufacturer’s recommendations for the CM5 chip and for the CMD500M chip.
|
|
CLOSE

|
Table 2.
Kinetic parameters and equilibrium dissociation constant of complexes of IgG2a with immobilized protein A.
|
FUNDING
The work was carried out within the framework of the Program for Basic Research in the Russian Federation for a long-term period (2021–2030) (No. 122030100168-2).
REFERENCES
- Karlsson, R. (2004) SPR for molecular interaction analysis: a review of emerging application areas. Journal of molecular recognition: JMR, 17(3), 151–161. DOI
- Jason-Moller, L., Murphy, M., Bruno, J. (2006) Overview of Biacore systems and their applications. Current Protocols in Protein Science, Chapter 19, Unit 19.13. DOI
- Florinskaya, A., Ershov, P., Mezentsev, Y., Kaluzhskiy, L., Yablokov, E., Medvedev, A., Ivanov, A. (2018) SPR biosensors in direct molecular fishing: implications for protein interactomics. Sensors, 18(5), 1616. DOI
- Liu, H., Fu, Y., Yang, R., Guo, J., Guo, J. (2023) Surface plasmonic biosensors: principles, designs and applications. The Analyst, 148(24), 6146–6160. DOI
- Douzi, B. (2024) Surface plasmon resonance: a sensitive tool to study protein-protein interactions. Methods in Molecular Biology (Clifton, N.J.), 2715, 363–382. DOI
- Yablokov, E.O., Sushko, T.A., Ershov, P.V., Florinskaya, A.V., Gnedenko, O.V., Shkel, T.V., Grabovec, I.P., Strushkevich, N.V., Kaluzhskiy, L.A., Usanov, S.A., Gilep, A.A., Ivanov, A.S. (2019) A large-scale comparative analysis of affinity, thermodynamics and functional characteristics of interactions of twelve cytochrome P450 isoforms and their redox partners. Biochimie, 162, 156–166. DOI
- Ershov, P.V., Kaluzhskiy, L.A., Yablokov, E.O., Gnedenko, O.V., Kavaleuski, A.A., Tumilovich, A.M., Gilep, A.A., Strushkevich, N.V., Ivanov, A.S. (2021) Application of the SPR biosensor for the analysis of protein–protein interactions in aqueous environment and bilayer lipid membrane as exemplified by P450scc (CYP11A1). Biochemistry (Moscow), Supplement Series A: Membrane and Cell Biology, 15(1), 89–96. DOI
- Kaluzhskiy, L., Ershov, P., Yablokov, E., Shkel, T., Grabovec, I., Mezentsev, Y., Gnedenko, O., Usanov, S., Shabunya, P., Fatykhava, S., Popov, A., Artyukov, A., Styshova, O., Gilep, A., Strushkevich, N., Ivanov, A. (2021) Human lanosterol 14-alpha demethylase (CYP51A1) is a putative target for natural flavonoid luteolin 7,3′-disulfate. Molecules, 26(8), 2237. DOI
- Ershov, P.V., Yablokov, E., Zgoda, V., Mezentsev, Y., Gnedenko, O., Kaluzhskiy, L., Svirid, A., Gilep, A., Usanov, S.A., Ivanov, A. (2021) A new insight into subinteractomes of functional antagonists: Thromboxane (CYP5A1) and prostacyclin (CYP8A1) synthases. Cell Biology International, 45(6), 1175–1182. DOI
- Gilep, A., Varaksa, T., Bukhdruker, S., Kavaleuski, A., Ryzhykau ,Y., Smolskaya, S., Sushko, T., Tsumoto, K., Grabovec, I., Kapranov, I., Okhrimenko, I., Marin, E., Shevtsov, M., Mishin, A., Kovalev, K., Kuklin, A., Gordeliy, V., Kaluzhskiy, L., Gnedenko, O., Yablokov, E., Ivanov, A., Borshchevski,y V., Strushkevich, N. (2023) Structural insights into 3Fe–4S ferredoxins diversity in M. tuberculosis highlighted by a first redox complex with P450. Frontiers in Molecular Biosciences, 9, 1100032. DOI
- Shepherd, C., Robinson, S., Berizzi, A., Thompson, L.E J., Bird, L., Culurgioni, S., Varzandeh, S., Rawlins, P.B., Olsen, R.H.J., Navratilova, I.H. (2022) Surface plasmon resonance screening to identify active and selective adenosine receptor binding fragments. ACS medicinal chemistry letters, 13(7), 1172–1181. DOI
- Zhang, X., Sun, L., Meuser, M.E., Zalloum, W.A., Xu, S., Huang, T., Cherukupalli, S., Jiang, X., Ding, X., Tao, Y., Kang, D., De Clercq, E., Pannecouque, C., Dick, A., Cocklin, S., Liu, X., Zhan, P. (2021) Design, synthesis, and mechanism study of dimerized phenylalanine derivatives as novel HIV-1 capsid inhibitors. European Journal of Medicinal Chemistry, 226, 113848. DOI
- Wang, W., Thiemann, S., Chen, Q. (2022) Utility of SPR technology in biotherapeutic development: Qualification for intended use. Analytical Biochemistry, 654, 114804. DOI
- Matharu, Z., Bee, C., Schwarz, F., Chen, H., Tomlinson, M., Wu, G., Rakestraw, G., Hornsby, M., Drake, A., Strop, P., Rajpal, A., Dollinger, G. (2021) High-throughput surface plasmon resonance biosensors for identifying diverse therapeutic monoclonal antibodies. Analytical Chemistry, 93(49), 16474–16480. DOI
- Zhu, Z.-L., Qiu, X.-D., Wu, S., Liu, Y.-T., Zhao, T., Sun, Z.-H., Li, Z.-R., Shan, G.-Z. (2020) Blocking Effect of Demethylzeylasteral on the Interaction between Human ACE2 Protein and SARS-CoV-2 RBD Protein Discovered Using SPR Technology. Molecules (Basel, Switzerland), 26(1), 57. DOI
- Risse, F., Gedig, E.T., Gutmann, J.S. (2018) Carbodiimide-mediated immobilization of acidic biomolecules on reversed-charge zwitterionic sensor chip surfaces. Analytical and Bioanalytical Chemistry, 410(17), 4109–4122. DOI
- Brown, M.E., Bedinger, D., Lilov, A., Rathanaswami, P., Vásquez, M., Durand, S., Wallace-Moyer, I., Zhong, L., Nett J. H., Burnina, I., Caffry, I., Lynaugh, H., Sinclair, M., Sun, T., Bukowski, J., Xu, Y., Abdiche, Y. N. (2020) Assessing the binding properties of the anti-PD-1 antibody landscape using label-free biosensors. PloS One, 15(3), e0229206. DOI
- Chen, Q., Manzke, M., Hartmann, A., Büttner, M., Amann, K., Pauly, D., Wiesener, M., Skerka, C., Zipfel, P.F. (2016) Complement factor H-related 5-hybrid proteins anchor properdin and activate complement at self-surfaces. Journal of the American Society of Nephrology, 27(5), 1413–1425. DOI
- Fu, Z., Xu, Y., Cai, C. (2021) Ginsenoside Rg3 inhibits pulmonary fibrosis by preventing HIF-1α nuclear localisation. BMC Pulmonary Medicine, 21(1), 70. DOI
- Di Primo, C., Dausse, E., Toulmé, J.-J. (2011) Surface plasmon resonance investigation of RNA aptamer–RNA ligand interactions. In J. Goodchild (Ed.), Therapeutic Oligonucleotides (Vol. 764, pp. 279–300). Totowa, NJ: Humana Press. DOI
- Desmyter, A., Farenc, C., Mahony, J., Spinelli, S., Bebeacua, C., Blangy, S., Veesler, D., Van Sinderen, D., Cambillau, C. (2013) Viral infection modulation and neutralization by camelid nanobodies. Proceedings of the National Academy of Sciences, 110(15). DOI
- Rohlik, D. L., Patel, E., Gilbert, N.C., Offenbacher, A.R., Garcia, B.L. (2023) Investigating membrane-binding properties of lipoxygenases using surface plasmon resonance. Biochemical and Biophysical Research Communications, 670, 47–54. DOI
- XanTec. Comparison between sensor chips from XanTec and other manufacturers. Newsletter II. Retrieved March 28, 2024, from: https://www.xantec.com/material/pdf/comparison_between_sensorchips_from_xantec_and_other_manufacturers.pdf
- Steinicke, F., Oltmann-Norden, I., Wätzig, H. (2017) Long term kinetic measurements revealing precision and general performance of surface plasmon resonance biosensors. Analytical Biochemistry, 530, 94–103. DOI