Efferocytosis as One of the Mechanisms for Realizing the Therapeutic Effects of Mesenchymal Stem Cells
Institute of Biomedical Chemistry, Pogodinskaya Street, 10, Moscow 119121, Russia; *e-mail: irkhol@yandex.ru
Keywords: efferocytosis; apoptosis; mesenchymal stem cells; inflammation; regeneration; immunomodulation
DOI:10.18097/BMCRM00221
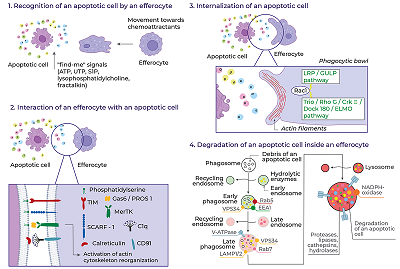
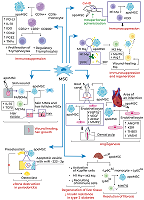
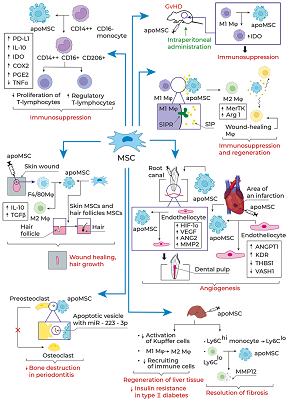
Mesenchymal stem cells (MSCs) stimulate regeneration and exhibit unique immunomodulatory properties, which makes them attractive for use in cell therapies of a wide range of pathologies. The clinical use of MSCs is hampered by the insufficiently clear understanding of their therapeutic action mechanisms. It has been reliably proven that MSCs after transplantation quickly die in the recipient’s body by the mechanism of apoptosis and are cleared by professional, such as macrophages, and non-professional phagocytes, including endothelial cells, hepatocytes, resident stem cells of various tissues, including MSCs. The ingestion and processing of apoptotic cells by the phagocytes was named efferocytosis. Despite rapid elimination of transplanted cells, in most cases MSC transplantation leads to positive therapeutic effects. Clearance of apoptotic MSCs affects phagocytes, changing their phenotype, secretome, and further behavior. This review presents the basic molecular mechanisms of efferocytosis, examines the clearance of apoptotic MSCs and their therapeutic effects in various pathologies in the context of their efferocytosis by various types of phagocytes.
FUNDING
The research was supported by the Russian Science Foundation grant No. 23-15-00149.
REFERENCES
- Fuchs, Y., Steller, H. (2011) Programmed cell death in animal development and disease. Cell, 147(4), 742–758. DOI
- Yeo, W., Gautier, J. (2004) Early neural cell death: Dying to become neurons. Dev. Biol. 274(2), 233–244. DOI
- Varela-Nieto, I., Palmero, I., Magariños, M. (2019) Complementary and distinct roles of autophagy, apoptosis and senescence during early inner ear development. Hear. Res., 376, 86–96. DOI
- Blume, Z.I., Lambert, J.M., Lovel, A.G., Mitchell, D.M. (2020) Microglia in the developing retina couple phagocytosis with the progression of apoptosis via P2RY12 signaling. Dev. Dyn., 249(6), 723–740. DOI
- Jahnukainen, K., Chrysis, D., Hou, M., Parvinen, M., Eksborg, S., Söder,O. (2004) Increased apoptosis occurring during the first wave of spermatogenesis is stage-specific and primarily affects midpachytene spermatocytes in the rat testis. Biol. Reprod., 70(2), 290–296. DOI
- Li, A., Felix, J.C., Hao, J., Minoo, P., Jain, J.K. (2005) Menstrual-like breakdown and apoptosis in human endometrial explants. Hum. Reprod., 20(6), 1709–1719. DOI
- Zhang, N., Hartig, H., Dzhagalov, I., Draper, D., He, Y.W. (2005) The role of apoptosis in the development and function of T lymphocytes. Cell Res., 15(10), 749–769. DOI
- Comi, C., Fleetwood, T., Dianzani, U. (2012)The role of T cell apoptosis in nervous system autoimmunity. Autoimmun. Rev., 12(2), 150–156. DOI
- Casamayor-Polo, L., López-Nevado, M., Paz-Artal, E., Anel, A., Rieux-Laucat, F., Allende, L.M. (2021) Immunologic evaluation and genetic defects of apoptosis in patients with autoimmune lymphoproliferative syndrome (ALPS). Crit. Rev. Clin. Lab. Sci., 58(4), 253–274. DOI
- Cassim, A., Hettiarachchi, D., Dissanayake, V.H.W. (2022) Genetic determinants of syndactyly: Perspectives on pathogenesis and diagnosis. Orphanet J. Rare Dis., 17(1), 198. DOI
- Avagliano, L., Doi, P., Tosi, D., Scagliotti, V., Gualtieri, A., Gaston-Massuet, C., Pistocchi, A., Gallina, A., Marconi, A.M., Bulfamante, G., Massa, V. (2016) Cell death and cell proliferation in human spina bifida. Birth Defects Res. A Clin. Mol. Teratol., 106(2), 104–113. DOI
- Pistritto, G., Trisciuoglio, D., Ceci, C., Garufi, A., d'Orazi, G. (2016) Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY), 8(4), 603–619. DOI
- Han, C.Z., Ravichandran, K.S. (2011) Metabolic connections during apoptotic cell engulfment. Cell, 147(7), 1442–1445. DOI
- Boada-Romero E., Martinez J., Heckmann B.L., Green D.R. (2020) The clearance of dead cells by efferocytosis. Nat. Rev. Mol. Cell Biol., 21(7), 398–414. DOI
- Juban, G., Chazaud, B. (2021) Efferocytosis during skeletal muscle regeneration. Cells, 10(12), 3267. DOI
- Kholodenko, I.V., Yarygin, K.N. (2023) Hepatic macrophages as targets for the MSC-based cell therapy in non-alcoholic steatohepatitis. Biomedicines, 11(11), 3056. DOI
- Wang, X., He, Q., Zhou, C., Xu, Y., Liu, D., Fujiwara, N., Kubota, N., Click, A., Henderson, P., Vancil, J., Marquez, C.A., Gunasekaran, G., Schwartz, M.E., Tabrizian, P., Sarpel, U., Fiel, M.I., Diao, Y., Sun, B., Hoshida, Y., Liang, S., Zhong, Z. (2023) Prolonged hypernutrition impairs TREM2-dependent efferocytosis to license chronic liver inflammation and NASH development. Immunity, 56(1), 58–77.e11. DOI
- Zhang, S., Weinberg, S., deBerge, M., Gainullina, A., Schipma, M., Kinchen, J.M., Ben-Sahra, I., Gius, D.R., Yvan-Charvet, L., Chandel, N.S., Schumacker, P.T., Thorp, E.B. (2019) Efferocytosis fuels requirements of fatty acid oxidation and the electron transport chain to polarize macrophages for tissue repair. Cell Metab., 29(2), 443–456.e5. DOI
- Brandel, V., Schimek, V., Göber, S., Hammond, T., Brunnthaler, L., Schrottmaier, W.C., Mussbacher, M., Sachet, M., Liang, Y.Y., Reipert, S., Ortmayr, G., Pereyra, D., Santol, J., Rainer, M., Walterskirchen, N., Ramos, C., Gerakopoulos, V., Rainer, C., Spittler, A., Weiss, T., Kain, R., Messner, B., Gruenberger, T., Assinger, A., Oehler, R., Starlinger, P. (2022) Hepatectomy-induced apoptotic extracellular vesicles stimulate neutrophils to secrete regenerative growth factors. J. Hepatol., 77(6), 1619–1630. DOI
- Kholodenko, I.V., Kholodenko, R.V., Majouga, A.G., Yarygin, K.N. (2022) Apoptotic MSCs and MSC-derived apoptotic bodies as new therapeutic tools. Curr. Issues Mol. Biol., 44(11), 5153–5172. DOI
- Preda, M.B., Neculachi, C.A., Fenyo, I.M., Vacaru, A.M., Publik, M.A., Simionescu, M., Burlacu, A. (2021) Short lifespan of syngeneic transplanted MSC is a consequence of in vivo apoptosis and immune cell recruitment in mice. Cell Death Dis., 12(6), 566. DOI
- Lu, W., Fu, C., Song, L., Yao, Y., Zhang, X., Chen, Z., Li, Y., Ma, G., Shen, C. (2013) Exposure to supernatants of macrophages that phagocytized dead mesenchymal stem cells improves hypoxic cardiomyocytes survival. Int. J. Cardiol., 165, 333–340. DOI
- Wagoner, Z.W., Zhao, W. (2021) Therapeutic implications of transplanted-cell death. Nat. Biomed. Eng., 5(5), 379–384. DOI
- Elliott, M.R., Koster, K.M., Murphy, P.S. (2017) Efferocytosis signaling in the regulation of macrophage inflammatory responses. J. Immunol., 198(4), 1387–1394. DOI
- Kelley, S.M., Ravichandran, K.S. (2021) Putting the brakes on phagocytosis: “Don't-eat-me” signaling in physiology and disease. EMBO Rep., 22(6), e52564. DOI
- Azuma, Y., Nakagawa, H., Dote, K., Higai, K., Matsumoto, K. (2011) Decreases in CD31 and CD47 levels on the cell surface during etoposide-induced Jurkat cell apoptosis. Biol. Pharm. Bull., 34(12), 1828–1834. DOI
- Tada, K., Tanaka, M., Hanayama, R., Miwa, K., Shinohara, A., Iwamatsu, A., Nagata, S. (2003) Tethering of apoptotic cells to phagocytes through binding of CD47 to Src homology 2 domain-bearing protein tyrosine phosphatase substrate-1. J. Immunol., 171(11), 5718–5726. DOI
- Lv, Z., Bian, Z., Shi, L., Niu, S., Ha, B., Tremblay, A., Li, L., Zhang, X., Paluszynski, J., Liu, M., Zen, K., Liu, Y. (2015) Loss of cell surface CD47 clustering formation and binding avidity to SIRPα facilitate apoptotic cell clearance by macrophages. J. Immunol., 195(2), 661–671. DOI
- Shi, H., Wang, X., Li, F., Gerlach, B.D., Yurdagul, A. Jr, Moore, M.P., Zeldin, S., Zhang, H., Cai, B., Zheng, Z., Valenti, L., Tabas, I. (2022) CD47-SIRPα axis blockade in NASH promotes necroptotic hepatocyte clearance by liver macrophages and decreases hepatic fibrosis. Sci. Transl. Med., 14(672), eabp8309. DOI
- Jarr, K.U., Kojima, Y., Weissman, I.L., Leeper, N.J. (2022) 2021 Jeffrey M. Hoeg Award lecture: Defining the role of efferocytosis in cardiovascular disease: A focus on the CD47 (Cluster of Differentiation 47) axis. Arterioscler. Thromb. Vasc. Biol., 42(6), e145–e154. DOI
- von Roemeling, C.A., Wang, Y., Qie, Y., Yuan, H., Zhao, H., Liu, X., Yang, Z., Yang, M., Deng, W., Bruno, K.A., Chan, C.K., Lee, A.S., Rosenfeld, S.S., Yun, K., Johnson, A.J., Mitchell, D.A., Jiang, W., Kim, B.Y.S. (2020) Therapeutic modulation of phagocytosis in glioblastoma can activate both innate and adaptive antitumour immunity. Nat. Commun., 11(1), 1508. DOI
- Zhang, J., Jin, S., Guo, X., Qian, W. (2018) Targeting the CD47-SIRPα signaling axis: Current studies on B-cell lymphoma immunotherapy. J. Int. Med. Res., 46(11), 4418–4426. DOI
- Xu, R., Xie, H., Shen, X., Huang, J., Zhang, H., Fu, Y., Zhang, P., Guo, S., Wang, D., Li, S., Zheng, K., Sun, W., Liu, L., Cheng, J., Jiang, H. (2023) Impaired efferocytosis enables apoptotic osteoblasts to escape osteoimmune surveillance during aging. Adv. Sci., 10(36), e2303946. DOI
- Bratton, D.L., Fadok, V.A., Richter, D.A., Kailey, J.M., Guthrie, L.A., Henson, P.M. (1997) Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J. Biol. Chem., 272(42), 26159–26165. DOI
- Birge, R.B., Boeltz, S., Kumar, S., Carlson, J.,Wanderley, J., Calianese, D., Barcinski, M., Brekken, R.A., Huang, X., Hutchins, J.T., Freimark, B., Empig, C., Mercer, J., Schroit, A.J., Schett, G., Herrmann, M. (2016) Phosphatidylserine is a global immunosuppressive signal in efferocytosis, infectious disease, and cancer. Cell Death Differ., 23(6), 962–978. DOI
- Gardai, S.J., McPhillips, K.A., Frasch, S.C., Janssen, W.J., Starefeldt, A., Murphy-Ullrich, J.E., Bratton, D.L., Oldenborg, P.A., Michalak, M., Henson, P.M. (2005) Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell, 123(2), 321–334. DOI
- Bohlson, S.S., Fraser, D.A., Tenner, A.J. (2006) Complement proteins C1q and MBL are pattern recognition molecules that signal immediate and long-term protective immune functions. Mol. Immunol., 44(1–3), 33–43. DOI
- Oroszlán, M., Daha, M.R., Cervenak, L., Prohászka, Z., Füst, G., Roos, A. (2007) MBL and C1q compete for interaction with human endothelial cells. Mol. Immunol., 44(6), 1150–1158. DOI
- Ogden, C.A., de Cathelineau, A., Hoffmann, P.R., Bratton, D., Ghebrehiwet, B., Fadok, V.A., Henson, P.M. (2001) C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med., 194(6), 781–795. DOI
- Meesmann, H.M., Fehr, E.M., Kierschke, S., Herrmann, M., Bilyy, R., Heyder, P., Blank, N., Krienke, S., Lorenz, H.M., Schiller, M. (2010) Decrease of sialic acid residues as an eat-me signal on the surface of apoptotic lymphocytes. J. Cell Sci., 123(Pt 19), 3347–3356. DOI
- Kinchen, J.M., Ravichandran, K.S. (2007) Journey to the grave: Signaling events regulating removal of apoptotic cells. J. Cell Sci., 120(Pt 13), 2143–2149. DOI
- Gutierrez, M.G. (2013) Functional role(s) of phagosomal Rab GTPases. Small GTPases, 4(3), 148–158. DOI
- Christoforidis, S., McBride, H.M., Burgoyne, R.D., Zerial, M. (1999) The Rab5 effector EEA1 is a core component of endosome docking. Nature, 397(6720), 621–625. DOI
- Fratti, R.A., Backer, J.M., Gruenberg, J., Corvera, S., Deretic, V. (2001) Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol., 154(3), 631–644. DOI
- Kaur, G., Lakkaraju, A. (2018) Early endosome morphology in health and disease. Adv. Exp. Med. Biol., 1074, 335–343. DOI
- Vieira, O.V., Botelho, R.J., Rameh, L., Brachmann, S.M., Matsuo, T., Davidson, H.W., Schreiber, A., Backer, J.M., Cantley, L.C., Grinstein, S. (2001) Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol., 155(1), 19–25. DOI
- Vieira, O.V., Bucci, C., Harrison, R.E., Trimble, W.S., Lanzetti, L., Gruenberg, J., Schreiber, A.D., Stahl, P.D., Grinstein, S. (2003) Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol. Cell. Biol., 23(7), 2501–2514. DOI
- Fairn, G.D., Grinstein, S. (2012) How nascent phagosomes mature to become phagolysosomes. Trends Immunol., 33(8), 397–405. DOI
- Kinchen, J.M., Ravichandran, K.S. (2008) Phagosome maturation: Going through the acid test. Nat. Rev. Mol. Cell Biol., 9(10), 781–795. DOI
- Huynh, K.K., Eskelinen, E.L., Scott, C.C., Malevanets, A., Saftig, P., Grinstein, S. (2007) LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J., 26(2), 313–324. DOI
- Dominici,M., le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., Deans, R., Keating, A., Prockop, Dj., Horwitz, E. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317. DOI
- Tsvetkova, A.V., Vakhrushev, I.V., Basok, Y.B., Grigor'ev, A.M., Kirsanova, L.A., Lupatov, A.Y., Sevastianov, V.I., Yarygin, K.N. (2021) Chondrogeneic potential of MSC from different sources in spheroid culture. Bull. Exp. Biol. Med., 170(4), 528–536. DOI
- Longhini, A.L.F., Salazar, T.E., Vieira, C., Trinh, T., Duan, Y., Pay, L.M., Li Calzi, S., Losh, M., Johnston, N.A., Xie, H., Kim, M., Hunt, R.J., Yoder, M.C., Santoro, D., McCarrel, T.M., Grant, M.B. (2019) Peripheral blood-derived mesenchymal stem cells demonstrate immunomodulatory potential for therapeutic use in horses. PLoS One, 14(3), e0212642. DOI
- Uzieliene, I., Bialaglovyte, P., Miksiunas, R., Lebedis, I., Pachaleva, J., Vaiciuleviciute, R., Ramanaviciene, A., Kvederas, G., Bernotiene, E. (2023) Menstrual blood-derived stem cell paracrine factors possess stimulatory effects on chondrogenesis in vitro and diminish the degradation of articular cartilage during osteoarthritis.Bioengineering (Basel), 10(9), 1001. DOI
- Lupatov, A.Y., Saryglar, R.Y., Vtorushina, V.V., Poltavtseva, R.A., Bystrykh, O.A., Chuprynin, V.D., Krechetova, L.V., Pavlovich, S.V., Yarygin, K.N., Sukhikh, G.T. (2021) Mesenchymal stromal cells isolated from ectopic but not eutopic endometrium display pronounced immunomodulatory activity in vitro. Biomedicines, 9(10), 1286. DOI
- Vakhrushev, I.V., Antonov, E.N., Popova, A.V., Konstantinova, E.V., Karalkin, P.A., Kholodenko, I.V., Lupatov,A.Y., Popov, V.K., Bagratashvili, V.N., Yarygin, K.N. (2012) Design of tissue engineering implants for bone tissue regeneration of the basis of new generation polylactoglycolide scaffolds and multipotent mesenchymal stem cells from human exfoliated deciduous teeth (SHED cells). Bull. Exp. Biol. Med., 153(1), 143–147. DOI
- Burunova, V.V., Gisina, A.M., Kholodenko, I.V., Lupatov, A.Y., Shragina, O.A., Yarygin, K.N. (2010) Standardization of biochemical profile of mesenchymal cell materials by probing the level of dehydrogenase activity. Bull. Exp. Biol. Med., 149(4), 497–501. DOI
- Miceli, V., Bulati, M., Iannolo, G., Zito, G., Gallo, A., Conaldi, P.G. (2021) Therapeutic properties of mesenchymal stromal/stem cells: The need of cell priming for cell-free therapies in regenerative medicine. Int. J. Mol. Sci., 22(2), 763. DOI
- Lotfy, A., AboQuella, N.M., Wang, H. (2023) Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther., 14(1), 66. DOI
- Krampera, M., le Blanc, K. (2021) Mesenchymal stromal cells: Putative microenvironmental modulators become cell therapy. Cell Stem Cell, 28(10), 1708–1725. DOI
- Yarygin, K.N., Namestnikova, D.D., Sukhinich, K.K., Gubskiy, I.L., Majouga, A.G., Kholodenko, I.V. (2021) Cell therapy of stroke: Do the intra-arterially transplanted mesenchymal stem cells cross the blood-brain barrier? Cells, 10(11), 2997. DOI
- Kholodenko, I.V., Yarygin, K.N., Gubsky, L.V., Konieva, A.A., Tairova, R.T., Povarova, O.V., Kholodenko, R.V., Burunova, V.V., Yarygin, V.N., Skvortsova, V.I. (2012) Intravenous xenotransplantation of human placental mesenchymal stem cells to rats: Comparative analysis of homing in rat brain in two models of experimental ischemic stroke. Bull. Exp. Biol. Med., 154(1), 118–123. DOI
- Giovannelli, L., Bari, E., Jommi, C., Tartara, F., Armocida, D., Garbossa, D., Cofano, F., Torre, M.L., Segale, L. (2023) Mesenchymal stem cell secretome and extracellular vesicles for neurodegenerative diseases: Risk-benefit profile and next steps for the market access. Bioact. Mater., 29, 16–35. DOI
- Xu, F., Fei, Z., Dai, H., Xu, J., Fan, Q., Shen, S., Zhang, Y., Ma, Q., Chu, J., Peng, F., Zhou, F., Liu, Z., Wang, C. (2022) Mesenchymal stem cell-derived extracellular vesicles with high PD-L1 expression for autoimmune diseases treatment. Adv. Mater., 34(1), e2106265. DOI
- Liu, H., Li, R., Liu, T., Yang, L., Yin, G., Xie, Q. (2020) Immunomodulatory effects of mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles in rheumatoid arthritis. Front. Immunol., 11, 1912. DOI
- Kholodenko, I.V., Kholodenko, R.V., Yarygin, K.N. (2023) The crosstalk between mesenchymal stromal/stem cells and hepatocytes in homeostasis and under stress. Int. J. Mol. Sci., 24(20), 15212. DOI
- Kholodenko, I.V., Kholodenko, R.V., Lupatov, A.Y., Yarygin, K.N. (2018) Cell therapy as a tool for induction of immunological tolerance after liver transplantation. Bull. Exp. Biol. Med., 165(4), 554–563. DOI
- Soontararak, S., Chow, L., Johnson, V., Coy, J., Wheat, W., Regan, D., Dow, S. (2018) Mesenchymal Stem Cells (MSC) derived from induced pluripotent stem cells (iPSC) equivalent to adipose-derived MSC in promoting intestinal healing and microbiome normalization in mouse inflammatory bowel disease model. Stem Cells Transl. Med., 7(6), 456–467. DOI
- Gnecchi, M., Danieli, P., Malpasso, G., Ciuffreda, M.C. (2016) Paracrine mechanisms of mesenchymal stem cells in tissue repair. Methods Mol. Biol., 1416, 123–146. DOI
- Eggenhofer, E., Luk, F., Dahlke, M.H., Hoogduijn, M.J. (2014) The life and fate of mesenchymal stem cells. Front. Immunol., 5, 148. DOI
- Pang, S.H.M., d'Rozario, J., Mendonca, S., Bhuvan, T., Payne, N.L., Zheng, D., Hisana, A., Wallis, G., Barugahare, A., Powell, D., Rautela, J., Huntington, N.D., Dewson, G., Huang, D.C.S., Gray, D.H.D., Heng, T.S.P. (2021) Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nat. Commun., 12(1), 6495. DOI
- Preda, M.B., Lupan, A.M., Neculachi, C.A., Leti, L.I., Fenyo, I.M., Popescu, S., Rusu, E.G., Marinescu, C.I., Simionescu, M., Burlacu, A. (2020) Evidence of mesenchymal stromal cell adaptation to local microenvironment following subcutaneous transplantation. J. Cell. Mol. Med., 24(18), 10889–10897. DOI
- Li, X., Jiang, Y., Liu, X., Fu, J., Du, J., Luo, Z., Xu, J., Bhawal, U.K., Liu, Y., Guo, L. (2023) Mesenchymal stem cell-derived apoptotic bodies alleviate alveolar bone destruction by regulating osteoclast differentiation and function. Int. J. Oral Sci., 15(1), 51. DOI
- Zhu, W., Chen, J., Cong, X., Hu, S., Chen, X. (2006) Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells, 24(2), 416–245. DOI
- Galleu, A., Riffo-Vasquez, Y., Trento, C., Lomas, C., Dolcetti, L., Cheung, T.S., von Bonin, M., Barbieri, L., Halai, K., Ward, S., Weng, L., Chakraverty, R., Lombardi, G., Watt, F.M., Orchard, K., Marks, D.I., Apperley, J., Bornhauser, M., Walczak, H., Bennett, C., Dazzi, F. (2017) Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med., 9(416), eaam7828. DOI
- Yamaza, T., Miura, Y., Bi, Y., Liu, Y., Akiyama, K., Sonoyama, W., Patel, V., Gutkind, S., Young, M., Gronthos, S., Le, A., Wang, C.Y., Chen, W., Shi, S. (2008) Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS ONE, 3(7), e2615. DOI
- Ham, O., Lee, S.Y., Song, B.W., Cha, M.J., Lee, C.Y., Park, J.H., Kim, I.K., Lee, J., Seo, H.H., Seung, M.J., Choi, E., Jang, Y., Hwang, K.C. (2015) Modulation of Fas-Fas ligand interaction rehabilitates hypoxia-induced apoptosis of mesenchymal stem cells in ischemic myocardium niche. Cell Transplant., 24(7), 1329–1341. DOI
- Kennea, N.L., Stratou, C., Naparus,A., Fisk, N.M., Mehmet,H. (2005) Functional intrinsic and extrinsic apoptotic pathways in human fetal mesenchymal stem cells. Cell Death Differ., 12(11), 1439–1441. DOI
- Manns, J., Daubrawa, M., Driessen, S., Paasch, F., Hoffmann, N., Löffler, A., Lauber, K., Dieterle, A., Alers, S., Iftner, T., Schulze-Osthoff, K., Stork, B., Wesselborg, S. (2011) Triggering of a novel intrinsic apoptosis pathway by the kinase inhibitor staurosporine: Activation of caspase-9 in the absence of Apaf-1. FASEB J., 25(9), 3250–3261. DOI
- Götherström, C., Lundqvist, A., Duprez, I.R., Childs, R., Berg, L., le Blanc, K. (2011) Fetal and adult multipotent mesenchymal stromal cells are killed by different pathways. Cytotherapy, 13(3), 269–278. DOI
- Kholodenko, I.V., Gisina, A.M., Manukyan, G.V., Majouga, A.G., Svirshchevskaya, E.V., Kholodenko, R.V., Yarygin, K.N. (2022) Resistance of human liver mesenchymal stem cells to FAS-induced cell death. Curr. Issues Mol. Biol., 44(8), 3428–3443. DOI
- Rodrigues, M., Turner, O., Stolz, D., Griffith, L.G., Wells, A. (2012) Production of reactive oxygen species by multipotent stromal cells/mesenchymal stem cells upon exposure to Fas ligand. Cell Transplant., 21(10), 2171–2187. DOI
- Solodeev, I., Meilik, B., Volovitz, I., Sela, M., Manheim, S., Yarkoni, S., Zipori, D., Gur, E., Shani, N. (2018) Fas-L promotes the stem cell potency of adipose-derived mesenchymal cells. Cell Death Dis., 9(6), 695. DOI
- Szegezdi, E., O'Reilly, A., Davy, Y., Vawda, R., Taylor, D.L., Murphy, M., Samali, A., Mehmet,H. (2009) Stem cells are resistant to TRAIL receptor-mediated apoptosis. J. Cell. Mol. Med., 13(11–12), 4409–4414. DOI
- Secchiero, P., Melloni, E., Corallini, F., Beltrami, A.P., Alviano, F., Milani, D., d'Aurizio, F., di Iasio, M.G., Cesselli, D., Bagnara, G.P., Zauli, G. (2008) Tumor necrosis factor-related apoptosis-inducing ligand promotes migration of human bone marrow multipotent stromal cells. Stem Cells, 26(11), 2955–2963. DOI
- Driscoll, P.C. (2014) Structural studies of death receptors. Methods Enzymol., 545, 201–242. DOI
- Mbongue, J.C., Nicholas, D.A., Torrez, T.W., Kim, N.S., Firek, A.F., Langridge, W.H. (2015) The role of indoleamine 2, 3-dioxygenase in immune suppression and autoimmunity. Vaccines (Basel), 3(3), 703–729. DOI
- Bikorimana, J.P., Saad, W., Abusarah, J., Lahrichi, M., Talbot, S., Shammaa, R., Rafei, M. (2022) CD146 defines a mesenchymal stromal cell subpopulation with enhanced suppressive properties. Cells, 11(15), 2263. DOI
- Liu, J., Qiu, X., Lv, Y., Zheng, C., Dong, Y., Dou, G., Zhu, B., Liu, A., Wang, W., Zhou, J., Liu, S., Liu, S., Gao, B., Jin, Y. (2020) Apoptotic bodies derived from mesenchymal stem cells promote cutaneous wound healing via regulating the functions of macrophages. Stem Cell Res. Ther., 11(1), 507. DOI
- Shapouri-Moghaddam, A., Mohammadian, S., Vazini, H., Taghadosi, M., Esmaeili, S.A., Mardani, F., Seifi, B., Mohammadi, A., Afshari, J.T., Sahebkar, A. (2018) Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol., 233(9), 6425–6440. DOI
- Yang, Z., Ming, X.F. (2014) Functions of arginase isoforms in macrophage inflammatory responses: Impact on cardiovascular diseases and metabolic disorders. Front. Immunol., 5, 533. DOI
- Shevela, E.Ya., Sakhno, L.V., Maksimova, A.A., Tikhonova, M.A., Ostanin, A.A., Chernykh, E.R. (2022) Expression of Arg1 and MerTK by human macrophages activated by M2-polarizing stimuli and their role in determining low allostimulatory activity. Immunologiya, 43(5), 515–524. DOI
- Weigert, A., Olesch, C., Brüne, B. (2019) Sphingosine-1- phosphate and macrophage biology — how the sphinx tames the big eater. Front. Immunol., 10, 1706. DOI
- Weichand, B., Weis, N., Weigert, A., Grossmann, N., Levkau, B., Brüne, B. (2013) Apoptotic cells enhance sphingosine-1-phosphate receptor 1 dependent macrophage migration. Eur. J. Immunol., 43(12), 3306–3313. DOI
- Luo, B., Gan, W., Liu, Z., Shen, Z., Wang, J., Shi, R., Liu, Y., Liu, Y., Jiang, M., Zhang, Z., Wu, Y. (2016) Erythropoeitin signaling in macrophages promotes dying cell clearance and immune tolerance. Immunity, 44(2), 287–302. DOI
- de Witte, S.F.H., Luk, F., Sierra Parraga, J.M., Gargesha, M., Merino, A., Korevaar, S.S., Shankar, A.S., O'Flynn, L., Elliman, S.J., Roy, D., Betjes, M.G.H., Newsome, P.N., Baan, C.C., Hoogduijn, M.J. (2018) Immunomodulation by therapeutic mesenchymal stromal cells (MSC) is triggered through phagocytosis of MSC by monocytic cells. Stem Cells, 36(4), 602–615. DOI
- Cheung, T.S., Galleu, A., von Bonin, M., Bornhäuser, M., Dazzi, F. (2019) Apoptotic mesenchymal stromal cells induce prostaglandin E2 in monocytes: Implications for the monitoring of mesenchymal stromal cell activity. Haematologica, 104(10), e438–e441. DOI
- Wang, R., Hao, M., Kou, X., Sui, B., Sanmillan, M.L., Zhang, X., Liu, D., Tian, J., Yu, W., Chen, C., Yang, R., Sun, L., Liu, Y., Giraudo, C., Rao, D.A., Shen, N., Shi, S. (2022) Apoptotic vesicles ameliorate lupus and arthritis via phosphatidylserinemediated modulation of T cell receptor signaling. Bioact. Mater., 25, 472–484. DOI
- Ma, L., Chen, C., Liu, D., Huang, Z., Li, J., Liu, H., Kin Kwok, R.T., Tang, B., Sui, B., Zhang, X., Tang, J., Mao, X., Huang, W., Shi, S., Kou, X. (2022) Apoptotic extracellular vesicles are metabolized regulators nurturing the skin and hair. Bioact. Mater., 19, 626–641. DOI
- Liu, H., Liu, S., Qiu, X., Yang, X., Bao, L., Pu, F., Liu, X., Li, C., Xuan, K., Zhou, J., Deng, Z., Liu, S., Jin, Y. (2020) Donor MSCs release apoptotic bodies to improve myocardial infarction via autophagy regulation in recipient cells. Autophagy, 16(12), 2140–2155. DOI
- Li, Z., Wu, M., Liu, S., Liu, X., Huan, Y., Ye, Q., Yang, X., Guo, H., Liu, A., Huang, X., Yang, X., Ding, F., Xu, H., Zhou, J., Liu, P., Liu, S., Jin, Y., Xuan, K. (2022) Apoptotic vesicles activate autophagy in recipient cells to induce angiogenesis and dental pulp regeneration. Mol. Ther., 30(10), 3193–3208. DOI
- Nussenzweig, S.C., Verma, S., Finkel, T. (2015) The role of autophagy in vascular biology. Circ. Res., 116(3), 480–488. DOI
- Liu, D., Kou, X., Chen, C., Liu, S., Liu, Y., Yu, W., Yu, T., Yang, R., Wang, R., Zhou, Y., Shi, S. (2018) Circulating apoptotic bodies maintain mesenchymal stem cell homeostasis and ameliorate osteopenia via transferring multiple cellular factors. Cell Res., 28(9), 918–933. DOI
- Zheng, C., Sui, B., Zhang, X., Hu, J., Chen, J., Liu, J., Wu, D., Ye, Q., Xiang, L., Qiu, X., Liu, S., Deng, Z., Zhou, J., Liu, S., Shi, S., Jin, Y. (2021) Apoptotic vesicles restore liver macrophage homeostasis to counteract type 2 diabetes. J. Extracell. Vesicles, 10(7), e12109. DOI
- Li, Y.H., Shen, S., Shao, T., Jin, M.T., Fan, D.D., Lin, A.F., Xiang, L.X., Shao, J.Z. (2021) Mesenchymal stem cells attenuate liver fibrosis by targeting Ly6Chi/lo macrophages through activating the cytokine-paracrine and apoptotic pathways. Cell Death Discov., 7(1), 239. DOI