|
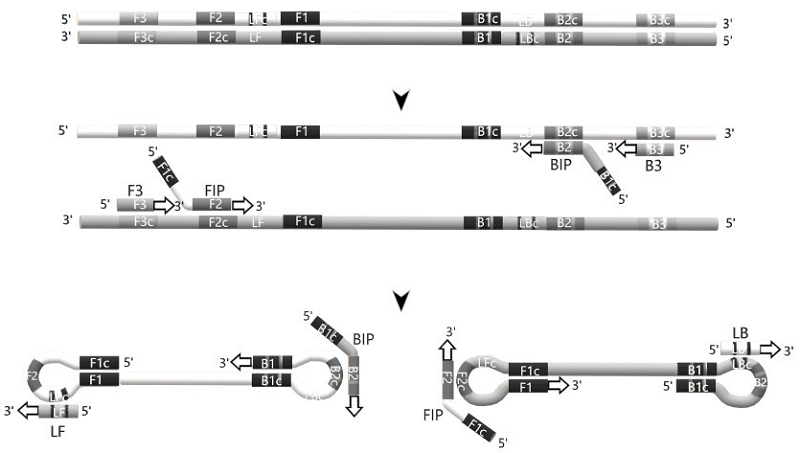
LAMP Primer Designing Software: The Overview 1Institute of Biomedical Chemistry, 10 Pogodinskaya str., Moscow, 119121 Russia; Key words: LAMP, primer design, software DOI: 10.18097/BMCRM00226 INTRODUCTION DNA diagnostics is a well-established approach to the identification of genetic, malignant, and infectious diseases: currently it heavily relies on the use of polymerase chain reaction (PCR). Although PCR undoubtedly remains the “gold standard” in DNA diagnostics, other methods based on the isothermal amplification of nucleic acids are steadily gaining an interest as an alternative to PCR, especially for testing in a “point-of-care” format [1]. Among them is the loop-mediated isothermal amplification (LAMP) developed in 2000 by Notomi et al. [2]. It is currently the most widely employed method for the isothermal amplification of DNA in both laboratory and point-of-care settings [3]. LAMP can specifically amplify a few copies of a DNA target by a factor of 109-1010 at a constant temperature of 60°C to 65°C in about 60 minutes, utilizing the strand-displacing DNA polymerase Bst (or its mutant variants Bst2.0 and Bst3.0, which are more processive, or another type of strand-displacing DNA polymerase, e.g., OmniAmp [4]) and two to three pairs of primers, one of which has a particular design [3, 5]. Alongside with fluorescence- or turbidity-based instrumental detection, the other detection modes compatible with the point-of-care format such as “naked-eye colorimetry” or the lateral flow assay were demonstrated to be suitable for visualizing the LAMP outcome [6]. Since LAMP is conducted at elevated temperatures in the range of 60-65°C, the primer annealing is governed by the similar thermodynamic rules as those in PCR. In PCR, the nearest-neighbor thermodynamic approach [7] appears to be the most accurate for melting temperature calculations and predicting the energetic stability of DNA structures (including that for the undesirable primer dimers formation) and served as a basis for developing software for PCR primers design [8]. However, in contrast to PCR primers, LAMP primers require a more elaborate design due to their multiplicity (up to six primers in contrast to merely two in PCR) as well as larger length and sophisticated sequence content of some of them [3]. Presently, there is a bunch of bioinformatic tools developed to construct LAMP primers, which are also based on algorithms taking into consideration the thermodynamics of primer/template duplexes. Along with the commercial products such as, e.g., LAMP Designers from OptiGene (www.optigene.co.uk/lamp-designer) and PREMIER Biosoft (www.premierbiosoft.com/isothermal/index.html), or Lamprim from Innova Plus (https://innovaplus.ru/products/programma-lamprim), there are programs for designing LAMP primers, which are freely available (at least, for the academic use). The present overview aims to provide a summary of these programs for an interested reader and to evaluate their merits and demerits. 1. SPECIFIC FEATURES OF LAMP PRIMERS As mentioned above, LAMP uses from two to three pairs of primers. Originally, LAMP was developed by employing two pairs of primers – one pair referred to as “outer primers” and another as “inner primers” [2, 3]. Later on, Nagamine et al. [5] modified the method by introducing an additional pair of primers (the so-called “loop primers”) to speed up the amplification reaction (the use of three primer pairs allowed for almost a twofold decrease in amplification time) [5]. Fig. 1 schematically illustrates LAMP principles. The forward and backward outer primers (FOP and BOP, respectively; also called F3 and B3) have a length of 18-20 nucleotides (nt) and are complementary to sequences flanking the targeted genomic region [3]. The forward and backward inner primers (FIP and BIP, respectively) are, in fact, the nested primers of a particular design. The FIP consists of a F2 region at the 3'end and a F1c region at the 5'end. The F2 region is complementary to the F2c region of the template sequence while the F1c region is identical to the F1c region of the template sequence (Fig. 1). The BIP primer is constructed in the similar manner (Fig. 1). The length of FIP and BIP primers is basically 40-42 nt [3]. The amplification reaction is initiated by the annealing of F3 and FIP primers to the corresponding regions of one DNA strand and B3 and BIP primers to another strand of the template (Fig. 1). Their elongation by a strand-displacing polymerase results in the occurrence of DNA single strands with hairpins formed on either 5’- or 3’-terminus due to annealing of F1 and F1c regions or B1 and B1c regions. The further annealing of FIP and BIP primers to corresponding sequences in the loop regions leads to the exponential production of stem-loop DNA structures with several inverted repeats of the target and cauliflower‐like structures with multiple loops [3] (Fig. 1). The length of amplified region – the distance from the 5’-end of F2 to the 5’-end of B2 – is usually between 120 to 180 base pairs (bp). The region defined by the outer primers should be about 300 bp or less, with the spacing between the inner and outer primers (including the length of the outer primers) of 40 bp or less [9]. The presence of loop primers (designated typically as LF and LB and ranging commonly from 18 to 25 nt in length) complementary to LoopFc and LoopBc regions (Fig. 1) in the reaction mixture increases the amplification rate. The recommended GC content for LAMP primers is from 40% to 60% but should preferably be between 50-60%. The primer melting temperature (Tm) has to be usually set at about 65°C (64-66°C) for F1c and B1c, about 60°C (59-61°C) for F2, B2, F3, and B3, and about 65°C (64 - 66°C) for the loop primers. If the AT-rich region is targeted, than Tm values should be decreased and lie between 55-60°C. The stability of 3’-end is another key feature in LAMP primer design similar to that as with the standard PCR primers [10]. Basically, the GC clamp (the presence of consecutive G or C bases within the 3'-end of primers defined as the last six bases of the primer’s 3’-terminal region) is strongly recommended. However, more than 3 consecutive G or C bases at the 3'-end of the primer have to be avoided. In overall, the 3’-ends of LAMP primers have to be designed so that the Gibb’s free energy change (the ΔG value) upon duplex formation is to be –4 kcal/mol or less. Moreover, formation of hairpin structures in primers can adversely affect the annealing. As a rule, the formation of a 3'-end hairpin with ΔG of –2 kcal/mol or more and an internal hairpin with ΔG of –3 kcal/mol or more are generally tolerated. 2. LAMP PRIMER DESIGN TOOLS To date, free bioinformatics tools for constructing LAMP primers have been developed. They include PrimerExplorer from the Eiken Chemical Co. (https://primerexplorer.jp/e/), NEB LAMP Primer Design Tool (NEB LAMP) from the New England Biolabs (https://lamp.neb.com/#!/), LAVA [11], GLAPD [12], LAMPrimer iQ [13], and MorphoCatcher [14] (the latter is freely available only for non-commercial use). Additionally, the FastPCR software [15] and the web tool STITCHER [16, 17] can be utilized for constructing LAMP primers. However, the FastPCR program can be downloaded from the PrimerDigital (https://primerdigital.com) for free use only within a limited trial period and so will be out of the scope of the present overview. The STITCHER resource appears optimal to design primers for overlapping (overlap extension) PCR but its application to the LAMP primer design would require the manual combining of generated primers in primer sets that can be quite time-consuming. Also, we will not discuss here any very specialized software aimed at adapting LAMP to particular applications, such as that developed, e.g., for the fluorescent probes design for LAMP assays to detect single nucleotide polymorphism (SNP) [18]. In addition to the bioinformatics tools mentioned above, the algorithm designated as eLAMP (electric LAMP) has been developed to predict the amplification success for a given set of LAMP primers and a target [19], similar to that as for PCR (e.g., [20-22]). Yet, the algorithm merely indicates, whether the amplification would proceed and is unable to simulate an amplification curve for a particular set of primers targeting the sequence of interest as in the case of some PCR simulators (e.g., [20]). Nonetheless, the script is available at https://www.nybg.org/files/scientists/dlittle/eLAMP.html for free download and can be used to check the selected LAMP primers. Additionally, to reduce false-positive LAMP results, mathematical models were developed to visualize the expected size pattern of specific LAMP product (e.g., an electrophoretic pattern) [23, 24]. PrimerExplorer Eiken’s PrimerExplorer is a free software specifically developed to design LAMP primers. Its latest version, the PrimerExplorer V5, has been released in 2016, although the previous version, the PrimerExplorer V4, still remains available at https://primerexplorer.jp/e/. Both versions possess the same set of functions, except for that the latest version additionally allows a user to save the primer set sequence information and to utilize the results of multiple sequence alignment (MSA) as an input (by clicking either the “Common” or ‘Specific” button). Once the user provides the input sequence and a set of design parameters, the program processes and displays a panel of primers considered as the most efficient by its algorithm. For a loaded target sequence, PrimerExplorer automatically determines its GC content. If GC content is between 45-60%, the default setting is used for primers design. When the target sequence is classified by the program as AT-rich (GC% <45) or GC-rich (GC% > 60), the primer design conditions (Tm, primers length, primers’ GC content) are automatically modified. There are two different primer design modes. The “Easy Mode” operates with predetermined parameters and displays five primer sets that are likely to have the highest amplification efficiencies. In this mode, the program uses the nearest-neighbor method to calculate melting temperatures at the fixed experimental conditions (oligo concentration of 0.1 µM, sodium ion concentration of 50 mM, magnesium ion concentration of 4 mM). The “Expert Mode” allows for the primer set customization by changing the parameters and the number of primers sets to be constructed. In both modes, the software selects first the basic (core) LAMP primers (F3, B3, FIP, and BIP) based on the six regions of the target sequence. Afterwards, the loop primers are selected in a separate execution, using the information on the selected core LAMP primers. During the primer design, the FIP-BIP pairs are first selected to cover the entire target region. Next, for each FIP-BIP region, F3 and B3 are selected to form a core primer set. The generation of primer sets by combining FIP-BIP with the F3 and B3 begins at the 5’-end of the target and proceeds until its 3’-end is reached. Each FIP- BIP pair can form a primer set with a maximum of three combinations of F3-B3. Of importance, PrimerExplorer has such feature as a possibility to take mutation locations into account. One can choose either the primer design function that does not include the mutation point or the function that allows the mutation to be included in the 5'-end, internally or in the 3’-end. One of the disadvantages of PrimerExplorer is the need to select loop primers in the additional session since that can complicate the identification of optimal primer combinations. If results turn out to be unsatisfactory, the user has to redesign the core primer set by varying the design parameters so that the entire procedure may become quite time-consuming. The program accepts sequences with length of only up to 2000 bp. Additionally, Primer Explorer allows no adjustments to be made to the automatically generated primer sets, thus posing significant restrictions on positioning of primers on the target. Moreover, a possibility to analyze the primer dimers formation is absent. NEB LAMP The algorithm for LAMP primer designing from the New England Biolabs, NEB LAMP, is freely available for online use at https://lamp.neb.com/#!/, starting from 2020, and based on principles similar to those of Eiken’s PrimerExplorer. However, in contrast to PrimerExplorer, after loading the DNA target sequence, the user has the option either to choose fixed positions for one of the LAMP primers on the entered template (in order to have a specific sequence inside or outside of the primer set region) or to simply proceed by clicking on the “Generate Primers” bottom to automatically select primer sets for the entire template. The candidate LAMP primer sets with their positions on the template are immediately displayed on the next screen and, in more details, in the table below. The table provides information on each primer’s Tm, length, and GC content, as well as the positions of primer’s 5’- and 3’-ends on the templates and their ΔG values. All sets of core LAMP primers are stored in the “Core Primers tab”. Upon selecting a core LAMP primer set, the user can initiate generation of loop primers for a particular set of core LAMP primers. The whole set of LAMP primers can be viewed in and downloaded from the “Result table”. Similar to PrimerExplorer, the primer design can be executed with either default parameters or adjusted parameters by using the functions of the “Preference tab”. Among the adjustable parameters are sodium and magnesium ion concentrations, primer lengths, Tm limits, primer GC content, ΔG threshold values (including those for the primer dimer stability), etc. Also, the particular default parameters can be set for AT- or GC-reach sequences by choosing corresponding options in the “Preference tab”. In any design mode, only the top 10 sets of core LAMP primers are displayed, ranked by their ΔG values. As with PrimerExplorer, the disadvantage of NEB LAMP is the selection of loop primers in a separate step. For some sets of core primers, no loop primers can be generated (in this case a warning will pop up), or merely one of the two loop primers can be suggested by the software. In such a case, the user has to return to the “Core Primers tab” and repeat the generation of loop primers for another set of core primers. Another disadvantage is that the length of target sequence is also limited by 2000 nt. LAVA The LAVA (LAMP Assay Versatile Analysis) algorithm was developed by Torrel et al. [11] in 2011 to overcome some of limitations of PrimerExplorer. LAVA was presented as a flexible tool for designing in one execution the complete candidate LAMP primer sets (LAMP signatures), each including both core and loop primers, free available for download at https://github.com/pseudogene/lava-dna?tab=readme-ov-file. In contrast to PrimerExplorer and NEB LAMP which use only a single sequence with a length of up to 2000 bp as an input, the algorithm could accept single sequences of up to 20000 bp in length, although on the expense of considerably long running time (up to 90 min on a desktop computer). Moreover, LAVA was able to process several sequences at once, which would be presented as MSA results precomputed by the BioPerl alignment module [25]. The BioPerl’s Primer3 module [25, 26] was utilized by the algorithm to separately run a search for each primer context for F1/B1, LF/LB, F2/B2, and F3/B3 primer pairs, using either the default or customized parameters and conservative regions of the allied sequences as a template. The primer pairs are filtered by their overlap, melting temperature, and the length. The combinations of primer pairs constituting LAMP signatures are then scored by assigning penalties, based on the size, primer spacing, Tm range. If the minimum number of signatures is not identified for the target, the entire primer combination process is repeated with different primer overlap cutoff percentages. Although LAVA could design a complete set of LAMP primers in a single execution for a conservative genomic region, it was still unable check the specificity of the set to ensure a design of group-specific primers. Moreover, LAVA lacked the capability to reject primers, which could form homo- and/or heterodimers in order to prevent unspecific amplification. Nonetheless, there are some studies, where LAVA was actually employed for selecting successful LAMP primer sets (e.g., [27-31]). Unfortunately, LAVA is not downloadable now (https://github.com/pseudogene/lava-dna?tab=readme-ov-file) and it has been discussed here as a milestone example in the development of software for LAMP primer design. GLAPD Genome based LAMP Primer Designer (GLAPD) is a program developed by Jia et al. in 2019 [12] to select LAMP primers for a group of target genomes by using the whole genomes as an input. Similar to LAVA, GLAPD first identifies the candidate single primer regions genome-wide and then combines selected primers into LAMP primer sets. Parameters and thresholds in GLAPD are similar to those used in PrimerExplorer V5. For a given group of target genomes, merely primer sets amplifying only conservative regions of these genomes will be the output. Designing LAMP primers, GLAPD uses two groups of genome sequences as an input: the target group and the background group. One genome from the target group is randomly selected as a reference genome. GLAPD identifies all candidate primer regions in the reference genome and generates primer sequences utilizing the nearest-neighbor thermodynamic approach, including a check on secondary structure of primers, similar to that as realized in the latest modifications of the Primer3 program [32, 33]. The primer’s ends are checked to exclude symmetric sequences and homopolymers. The customized parameters can be used to identify target regions. The Tm values of primers are automatically adjusted to whether the target regions are AT- or GC-reach. Primers from six regions (F3, F2, F1c, B1c, B2, and B3) are combined by GLAPD into one basic LAMP primer set, based on their positional relationship, GC content, and Tm values (the Tm values of F1c and B1c are 3°C higher than those of other primers). The LAMP primer set should be able to amplify all genomes of the target group (commonality) and no even single genome from the background group (specificity). To check the commonality and specificity, all single primers are aligned to the target and background genomes, respectively, using the Bowtie aligner [34]. By default, no mismatch in a primer is allowed for the target group. If GLAPD fails to design such LAMP primer set, a small number of mismatches are allowed. However, if a primer can be aligned to a background genome even with two mismatches (by default), this primer is considered as non-specific and discharged. By default, GLAPD can design up to 10 LAMP primer sets. Furthermore, GLAPD can also design LAMP primer set with loop primers. The candidate primer regions are identified for loop primers from the reference genome first. Then, GLAPD combines loop primers with other single primers into a complete LAMP primer set. A complete LAMP primer set can contain one or two loop primers. GLAPD can be accessed for free download at http://cgm.sjtu.edu.cn/GLAPD/ or for online design at https://cgm.sjtu.edu.cn/GLAPD/online/ and appears to meet many requirements for a construction of successful LAMP primers. Yet, up to now, this algorithm was rather rarely employed to actually construct LAMP primers, compared with PrimerExplorer or NEB LAMP. Nevertheless, being used for designing the group-specific LAMP primers, GLAPD demonstrated capability to provide successful primer sets for practical applications (e.g., [35-38]). LAMPrimer iQ The LAMPrimer iQ algorithm has been developed in 2024 by Ahmetzaianova et al. [13] and is freely available as SaaS (Software as a Service) as described at https://github.com/Restily/LAMPrimers-iQ. The developers specifically emphasize that the relatively large (compared to PCR) number of primers necessary to conduct LAMP increases the probability of primer dimer formation. That in turn can often result in nonspecific amplification compromising the reliability of the LAMP assay. The LAMPrimer iQ algorithm includes special steps to ensure that the design of LAMP primers is carried with stringent selection criteria to reject primers, which can form homo- and/or heterodimers (overlapping of two or more 3’-terminal nucleotides is not allowed). To compensate for that such stringent criteria of primer selection can cause no primer sets generated, the algorithm allows for processing a long sequence (up to 15 million nt) at once. Another important feature of LAMPrimer iQ is that distances between the F1 and B1, F2 and F3, B2 and B3 recognition sites are reduced: the size of the targeted region is about 150 nt (compared to 200-300 nt for conventional LAMP primers). The underlying rational was that in PCR the proximity of primers appears to decrease nonspecific amplification [39, 40]. The Tm values for primers are calculated based on the nearest-neighbor thermodynamic approach though with a slight modification. The present version of LAMPrimer iQ merely provides sets of core LAMP primers, with no option to design loop primers. Moreover, MSA results cannot be used as an input. As the LAMPrimer iQ developers claim [13], these options as well as a built-in capability to perform BLAST analysis of generated primer sets are planned to be introduced in further versions of the software. Since the LAMPrimer iQ algorithm has become available only very recently, its ability to successfully design LAMP primers for practical applications is to be seen. It is also worth mentioning that LAMPrimer iQ appears to present the further development of the earlier program for LAMP primer design, based on the Aho-Karasik algorithm, by that research group [41]. MorphoCatcher The MorphoCatcher is an online plugin tool for the PrimerExplorer software that extends its capability for processing MSA composed from orthologous nucleotide sequences (e.g., bacterial or eukaryotic genes) or whole-genome sequences of viruses with conservative genome architecture. The plugin was developed in 2019 by Shirshikov et al. [14] and can be accessed for online use at http://morphocatcher.ru or free downloaded from https://github.com/shrshkv/MorphoCatcher as a command line Python script. The underlying idea was to select target sequence using a large multi-species alignment, identify all taxon-specific mutations and SNP sites, and use such specific mutations and SNPs as a main target for specific primer design option of PrimerExplorer. The algorithm is based on the finding of all taxon-specific mutations and SNPs (excluding strain-specific polymorphisms), which correspond to each taxon (or group) in multi-species and multi-strain MSA. Then, a sliding-window function is used to visualize the polymorphism density of potential target for each taxon of the MSA. That allows to select the target region with a high density of taxon-specific mutations, thus accelerating the target selection process. In the suggested protocol [14], the user has to collect first several strains of target species and the most closely related sequences from other species to form groups of strains, which are to be differentiated by the LAMP assay. Secondly, a set of potential targets should be selected in the taxon of interest (by using tools for comparative gene content analyses such as, e.g., EDGAR [42] or others, as well as various NCBI nucleotide databases) with a size preferably more than 1000 bp. Also, sequences have to be extracted from other closely related taxa for the statistically reliable identification of taxon-specific SNPs and mutations. The multi-species MSA analysis of the selected sequences is recommended to perform with the Cluster Omega algorithm [43] that has no significant restriction on the input sequence length. The MSA results are processed by MorphoCatcher with the output containing the following files: MSA with highlighted species-specific mutations; sliding-window plot of mutation density for each species of the alignment; ready-to-use file for design of specific primers, using PrimerExplorer. For the later, the corresponding text file from the ‘Input for PrimerExplorer’ column can be directly submitted to the PrimerExplorer V5 service. However, to overcome the problem of the limited target sequence size, well known for PrimerExplorer (≤ 2000 bp), the user should evaluate preliminary the polymorphism level of target sequence and after that select a suitable region (up to 2000 bp) with high density of taxon-specific mutations for primer design. Moreover, the problem of primer dimer retains since PrimerExplorer lacks the capability to discriminate primers prone to form homo- and/or heteroduplexes. CONCLUSIONS Currently, the free software for LAMP primer design is presented by several algorithms available as an online service and/or downloadable programs. Among them, PrimerExplorer is mostly used, followed by NEB LAMP, which is steadily gaining popularity, despite the fact that they both have some obvious limitations. The other non-commercial programs have been employed for LAMP primer design much more rarely. Compared to PrimerExplorer or NEB LAMP, these non-commercial programs offer possibility to use MSA results composed of long (up to genome size) sequences as an input, first of all. To become a free robust alternative to commercial programs for LAMP primer design, the further development of the non-commercial software has to be focused on a program or online service integrating the options of generating the complete sets of LAMP primers (including loop primers) in a single execution, alongside with the effective discrimination of primers capable to form primer dimers and, in ideal, with a check of primer specificity by using a built-in capability to perform BLAST analysis. COMPLIANCE WITH ETHICAL STANDARDS This article does not contain any research involving humans or using animals as objects. FUNDING The study was performed within the framework of the Program for Basic Research in the Russian Federation for a long-term period (2021–2030) (No. 122030100170-5). CONFLICT OF INTEREST The authors declare no conflict of interest. REFERENCES
|