Immobilization of L-Asparaginase on Oxidized Bacterial Cellulose to Improve the Thermal Stability of the Enzyme
Institute of Biomedical Chemistry, 10 Pogodinskaya str., Moscow, 119121 Russia; *e-mail: a.shishparyonok@yandex.ru
Keywords: L-asparaginase; bacterial cellulose; immobilization; thermal stability
DOI:10.18097/BMCRM00234
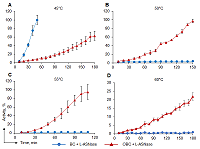
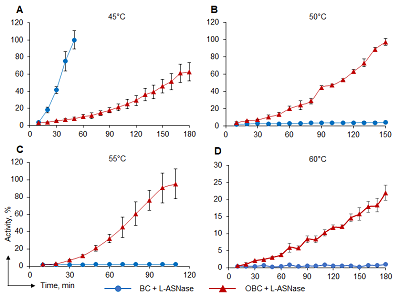
Bacterial cellulose (BC) membranes can be modified for covalent immobilization of macromolecules. One type of modification is oxidation, after which the oxidized BC membrane (OBC) could be used as a matrix for covalent immobilization of enzymes. In this work, the BC membrane was chemically oxidized with sodium periodate (NaIO4) to increase the stability of immobilized mesophilic L-asparaginase (L-ASNase) from Erwinia carotovora (EwA). IR spectroscopy confirmed the immobilization of L-ASNase EwA on OBC membranes. Immobilization of the enzyme increased its temperature optimum for its activity by 15°C and raised the inactivation temperature to 60°C. The OBC membrane could be used as a potential carrier for covalent immobilization of enzymes to improve their pharmacological properties by increasing their thermostability.
|
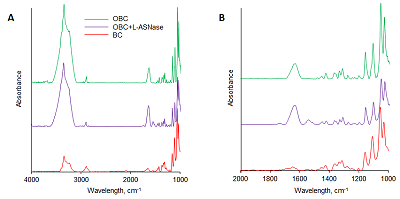
Figure 1. IR spectra of BC, OBC, and OBC films with immobilized L-ASNase EwA. (A) Spectral range 4000-1000 cm-1. (B) Spectral range 2000-1000 cm-1
|
|
CLOSE

|
Table 1.
Physicochemical characteristics of BC and OBC.
|
FUNDING
The work was conducted in accordance with framework of the Russian Federation fundamental research program for the long-term period (2021-2030) (No. 122022800499-5).
REFERENCES
- Aleksandrova, S.S., Abakumova, O.Yu., Podobed, O.V., Melik-Nubarov, N.S., Kudryashova, E.V., Grishin, D.V., Archakov, A.I. (2015) Bacterial recombinant L-asparaginases: Properties, structure and anti-proliferative activity. Biomeditsinskaya Khimiya, 61(3), 312–324. DOI
- Alexandrova, S.S., Gladilina, Y.A., Pokrovskaya, M.V., Sokolov, N.N., Zhdanov, D.D. (2022) Mechanisms of development of side effects and drug resistance to asparaginase and ways to overcome them. Biomeditsinskaya Khimiya, 68(2), 104–116. DOI
- Dumina, M.V., Eldarov, M.A., Zdanov, D.D., Sokolov, N.N. (2020) L-asparaginases of extremophilic microorganisms in biomedicine. Biomeditsinskaya Khimiya, 66(2), 105–123. DOI
- Tsegaye, K., Tsehai, B.A., Getie, B. (2024) Desirable L-asparaginases for treating cancer and current research trends. Front. Microbiol., 15, 1269282. DOI
- Darnal, S., Patial, V., Kumar, V., Kumar, S., Kumar, V., Padwad, Y.S., Singh D. (2023) Biochemical characterization of extremozyme L-asparaginase from Pseudomonas sp. PCH199 for therapeutics. AMB Express, 13(1), 22. DOI
- Papageorgiou, A.C., Posypanova, G.A., Andersson, S.A. Sokolov, N.N., Krasotkina, J. (2008) Structural and functional insights into Erwinia carotovora L-asparaginase. FEBS J., 275(17), 4306–4316. DOI
- Zhang, W., Dai, Q., Huang, Z., Xu, W. (2023) Identification and thermostability modification of the mesophilic L-asparaginase from Limosilactobacillus secaliphilus. Appl. Biochem. Biotechnol., 196(6), 3387–3401. DOI
- Jiao, L., Chi, H., Xia, B., Lu, Z., Bie, X., Zhao, H., Lu, F., Chen, M. (2022) Thermostability improvement of L-asparaginase from Acinetobacter soli via consensus-designed cysteine residue substitution. Molecules, 27(19), 6670. DOI
- Shishparenok, A.N., Gladilina, Y.A., Zhdanov, D.D. (2023) Engineering and expression strategies for optimization of L-asparaginase development and production. Int. J. Mol. Sci., 24(20), 15220. DOI
- Kotzia, G.A., Labrou, N.E. (2009) Engineering thermal stability of L-asparaginase by in vitro directed evolution. FEBS J., 276(6), 1750–1761. DOI
- Chi, H., Wang, Y., Xia, B., Zhou, Y., Lu, Z., Lu, F., Zhu, P. (2022) Enhanced thermostability and molecular insights for L-asparaginase from Bacillus licheniformis via structure- and computation-based rational design. J. Agric. Food Chem., 70(45), 14499–14509. DOI
- Bjørk, A., Dalhus, B., Mantzilas, D., Sirevåg, R., Eijsink, V.G.H. (2004) Large improvement in the thermal stability of a tetrameric malate dehydrogenase by single point mutations at the dimer-dimer interface. J. Mol. Biol., 341(5), 1215–1226. DOI
- Meneguetti, G.P., Santos, J.H.P.M., Obreque, K.M.T., Barbosa, C.M.V., Monteiro, G., Farsky, S.H.P., Marim de Oliveira, A., Angeli, C.B., Palmisano, G., Ventura, S.P.M., Pessoa-Junior, A., de Oliveira Rangel-Yagui, C. (2019) Novel site-specific PEGylated L-asparaginase. PloS ONE, 14(2), e0211951. DOI
- Feenstra, L.R., Gehring, R., van Geijlswijk, I.M., König, T., Prinsen, H.C.M.T., Vandemeulebroecke, K., Lammens, T., Krupa, A., Teske, E. (2022) Evaluation of PEG-L-asparaginase in asparagine suppression and anti-drug antibody development in healthy Beagle dogs: A multi-phase preclinical study. Veterinary J., 286, 105854. DOI
- Melik-Nubarov, N.S., Grozdova, I.D., Lomakina, G.Y., Pokrovskaya, M.V., Pokrovski, V.S., Aleksandrova, S.S., Abakumova, O.Y., Podobed, O.V., Grishin, D.V., Sokolov, N.N. (2017) PEGylated recombinant L-asparaginase from Erwinia carotovora: Production, properties, and potential applications. Appl. Biochem. Microbiol., 53(2), 165–172. DOI
- Vasconcelos, N.F., Andrade, F.K., Vieira, L.D.A.P., Vieira, R.S., Vaz J.M., Chevallier, P., Mantovani, D., Borges, M.D.F., Rosa, M.D.F. (2020) Oxidized bacterial cellulose membrane as support for enzyme immobilization: Properties and morphological features. Cellulose, 27(6), 3055–3083. DOI
- Wu, S.-C., Wu, S.-M., Su, F.-M. (2017) Novel process for immobilizing an enzyme on a bacterial cellulose membrane through repeated absorption. J. Chem. Technol. Biotechnol., 92(1), 109–114. DOI
- Meister, A. (1955) Glutaminase, asparaginase, and α-keto acid-ω-amidase. Methods Enzymol., 2, 380–385.
- Krasotkina, J., Borisova, A.A., Gervaziev, Y.V., Sokolov, N.N. (2004) One-step purification and kinetic properties of the recombinant L-asparaginase from Erwinia carotovora. Biotechnol. Appl. Biochem., 39(2), 215–221. DOI
- Bora, U., Kannan, K., Nahar, P. (2005) A simple method for functionalization of cellulose membrane for covalent immobilization of biomolecules. J. Membrane Sci., 250(1–2), 215–222. DOI
- Cai, Q., Hu, C., Yang, N., Wang, Q., Wang, J., Pan, H., Hu, Y., Ruan, C. (2018) Enhanced activity and stability of industrial lipases immobilized onto spherelike bacterial cellulose. Int. J. Biol. Macromol., 109, 1174–1181. DOI
- Drozd, R., Szymańska, M., Rakoczy, R., Junka, A., Szymczyk, P., Fijałkowski, K. (2019) Functionalized magnetic bacterial cellulose beads as carrier for Lecitase® Ultra immobilization. Appl. Biochem. Biotechnol., 187(1), 176–193. DOI
- Li, G., Nandgaonkar, A.G., Wang, Q., Zhang, J., Krause, W.E., Wei, Q., Lucia, L.A. (2017) Laccase-immobilized bacterial cellulose/TiO2 functionalized composite membranes: Evaluation for photo- and bio-catalytic dye degradation. J. Membrane Sci., 525(6), 89–98. DOI
- Li, J., Wan, Y., Li, L., Liang, H., Wang, J. (2009) Preparation and characterization of 2,3-dialdehyde bacterial cellulose for potential biodegradable tissue engineering scaffolds. Mater. Sci. Eng.: C, 29(5), 1635–1642. DOI
- Isobe, N., Lee, D.-S., Kwon, Y.-J., Kimura, S., Kuga, S., Wada, M., Kim, U.-J. (2011) Immobilization of protein on cellulose hydrogel. Cellulose, 18(5), 1251–1256. DOI
- Kumari, S., Chauhan, G.S., Ahn, J.-H., Reddy, N.S. (2016) Bio-waste derived dialdehyde cellulose ethers as supports for α-chymotrypsin immobilization. Int. J. Biol. Macromol., 85, 227–237. DOI
- Navapour, L., Mogharrab, N., Amininasab, M. (2014) How modification of accessible lysines to phenylalanine modulates the structural and functional properties of horseradish peroxidase: A simulation study. PLoS ONE, 9(10), e109062. DOI
- Kuchumova A.V. (2007) Pegilirovanie rekombinantnoj L-asparaginazy Erwinia carotovora s cel'yu usileniya ee terapevticheski znachimyh svojstv. Diss. kand. nauk, Institute of Biomedical Chemistry, Moscow.