Interaction of Mouse and Sheep Polyclonal Antibodies with the Main Forms of Human and Rat Renalase
Institute of Biomedical Chemistry, 10 Pogodinskaya str., Moscow, 119121 Russia; *e-mail: valfed38@yandex.ru
Keywords: polyclonal antibodies; human renalase-1 (RNLS1-human); rat renalase-2 (RNLS2-rat)
DOI:10.18097/BMCRM00248
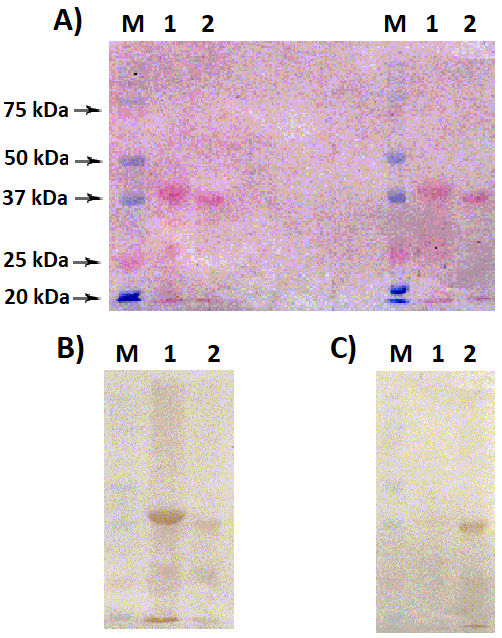
The interaction of sheep and mouse polyclonal antirenalase antibodies obtained by immunization with full-length human (RNLS1-human) and rat (RNLS2-rat) renalases, respectively, has been studied. The target recombinant proteins, RNLS1-human and RNLS2-rat, were expressed in E. coli cells and isolated by Ni-agarose chromatography. Sheep polyclonal antibodies against RNLS1-human interacted more effectively with both RNLS1-human than with RNLS2-rat. Mouse polyclonal antibodies against RNLS2-rat effectively interacted mainly with RNLS2-rat, but not with RNLS1-human. The data obtained indicate the preferential selectivity of the antibody interaction with the proteins against which they were obtained. This should be taken into consideration in the case of selection of commercially available antibody preparations for quantitative immunodetection of target proteins in biological objects.
FUNDING
The work was performed within the framework of the Program for Basic Research in the Russian Federation for a long-term period (2021-2030) (№ 122030100170-5).
REFERENCES
- Xu, J., Li, G., Wang, P., Velazquez, H., Yao, X., Li, Y., Wu, Y., Peixoto, A., Crowley, S., Desir, G.V. (2005) Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J. Clin. Invest., 115(5), 1275–1280. DOI
- Severina, I.S., Fedchenko, V.I., Veselovsky, A.V., Medvedev, A.E. (2015)The history of renalase from amine oxidase to a α-NAD(P)H-oxidase/anomerase. Biomeditsinskaya Khimiya. 61(6), 667-679. DOI
- Pointer, T.C., Gorelick, F.S., Desir, G.V. (2021) Renalase: a multi-functional signaling molecule with roles in gastrointestinal disease, Cells, 10, 2006. DOI
- Moran, G.R., Hoag, M.R. (2017) The enzyme: Renalase. Arch. Biochem. Biophys, 632, 66-76. DOI
- Fedchenko, V., Kopylov, A., Kozlova, N., Buneeva, O., Kaloshin, A., Zgoda, V., Medvedev, A. (2016) Renalase secreted by human kidney HEK293T cells lacks its n-terminal peptide: implications for putative mechanisms of renalase action, Kidney Blood Press Res. 41, 593-603. DOI
- Wang, Y., Safirstein, R., Velazquez, H., Guo, X.J., Hollander, L., Chang, J., Chen, T.M., Mu, J.J., Desir, G.V. (2017) Extracellular renalase protects cells and organs by outside-in signalling. J. Cell Mol. Med., 21(7), 1260-1265. DOI
- Kolodecik, T.R., Reed, A.M., Date, K., Shugrue, C.A., Patel, V., Chung, S.L., Desir, G.V., Gorelick, F.S. (2017) The serum protein renalase reduces injury in experimental pancreatitis. J. Biol. Chem., 292(51), 21047–21059. DOI
- Zhan,g L., Zang, C.S., Chen, B., Wang, Y., Xue, S., Wu, M.Y. (2023) Renalase regulates renal tubular injury in diabetic nephropathy via the p38MAPK signaling pathway. FASEB J. 37(10), e23188. DOI
- Retrieved 01.10.2024 from https://www.abcam.com/en-us/products/primary-antibodies/renalase-antibody-ab31291
- Fedchenko, V.I., Kaloshin, A.A., Mezhevikina, L.M., Buneeva, O.A., Medvedev, A.E. (2013) Construction of the coding sequence of the transcription variant 2 of the human Renalase gene and its expression in the prokaryotic system. Int. J. Mol. Sci.,14(6),12764-1279. DOI
- Fedchenko, V.I., Kaloshin, A.A. (2019) A simplified method for obtaining cDNA of low-copy and silent eukaryotic genes using human renalase as an example. Biomedical Chemistry: Research and Methods. 2(2), e00101. DOI
- Fedchenko, V., Kaloshin, A., Medvedev, A. (2023). Improvement of the exon method for rapid synthesis of cDNA of the rat renalase gene. Biomedical Chemistry: Research and Methods, 6(3), e00201. DOI
- Fedchenko, V., Kaloshin, A., Medvedev, A. (2024). Generation of C-terminal sequences of human renalase-1 and renalase-2 encoded by alternative exons. Biomedical Chemistry: Research and Methods, 7(2), e00228. DOI
- Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680-685. DOI
- Gallagher, S., Winston, S.E., Fuller, S.A., Hurrell, J.G. (2008) Immunoblotting and immunodetection. Curr. Protoc. Mol. Biol., Chapter 10:Unit 10.8. DOI
- Fedchenko, V.I., Veselovsky, A.V., Kopylov, A.T., Kaloshina, S.A., Medvedev, A.E. (2022) Renalase may be cleaved in blood. Are blood chymotrypsin-like enzymes involved? Medical Hypotheses, 165, 110895. DOI
- Fedchenko, V., Globa, A., Buneeva, O., Medvedev, A. (2013) Renalase mRNA levels in the brain, heart, and kidneys of spontaneously hypertensive rats with moderate and high hypertension. Med. Sci. Monit. Basic Res. 19, 267-270. DOI
- Retrieved 01.10.2024 from https://blast.ncbi.nlm.nih.gov/Blast.cgi.