Spectrophotometric Determination of the Ionization Constants for the Oximes – Cholinesterases Reactivators at Different Temperatures
State Scientific Research Test Institute of the Military Medicine, 4 Lesoparkovaya str., Saint-Petersburg, 195043 Russia; *e-mail: schafer@yandex.ru
Keywords:oximes; absorbtion spectra; protolytic dissotiation; ionization constants; temperature effect
DOI:10.18097/BMCRM00097
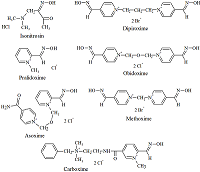
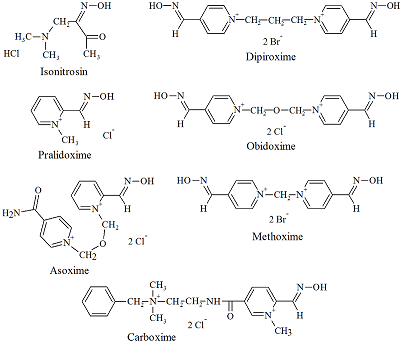
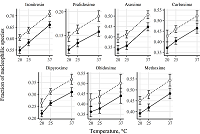
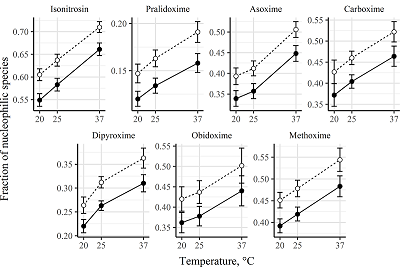
The goal of study was the determination of the ionization constants for the oximes – cholinesterases reactivators in aqueous solutions at different temperatures. The wavelengths of absorption maxima of the protonated and deprotonated oxime groups, the molar extinction coefficients of the various oximes species, and the ionization constants for the oxime cholinesterases reactivators (isonitrosin, pralidoxime, dipyroxime, toxogonin, methoxime, carboxime and asoxime) were obtained using spectrophotometric data (wavelength 190 to 450 nm) in solutions (pH 5-12) at 20°C, 25°C and 37°C. The proportion of nucleophilic forms involved in the oxime-induced reactivation of phosphorylated cholinesterases was shown to be is positively dependent on the incubation medium pH value and temperature. A hypothesis that the temperature affects the oximes ability to reactivate phosphorylated cholinesterases has been proposed.
|
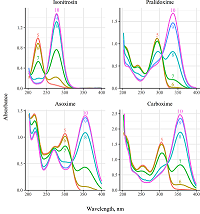
Figure 2.
Absorbance spectra of monooximes (1.0·10−4 M, 37°C) in solutions with various pH (shown as numbers inside plots).
|
|
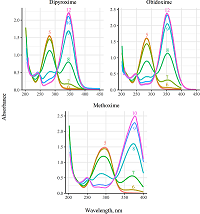
Figure 3.
Absorbance spectra of bispyridinium dialdoximes (5.0·10−5 М, 37°C) in solutions with various pH (shown as numbers inside plots).
|
|
CLOSE

|
Table 1.
Wavelengths corresponding to absorption maxima (λmax) of protonated and deprotonated oxime groups, nm
|
|
CLOSE

|
Table 2.
Molar absorption coefficients ε species of monooximes at at λmax of oximate-ion and ionization constants in aqueous solutions at different temperatures, (mean ± SEM)
|
|
CLOSE

|
Table 3.
Molar absorption coefficients ε species of bispyridinium dialdoximes at λmax of oximate-ion and ionization constants in aqueous solutions at different temperatures, (mean ± SEM)
|
|
CLOSE

|
Table 4.
Linear models characteristics of temperature dependence of the oximes pKa, pKa1, pKa2 values
|
REFERENCES
- Golikov, S. N. & Zaugolnikov S. D. (1970). Reactivators of cholinestherases. Leningrad, Medicina. [in Russian].
- Albert, A. & Serjeant, E. P. (1971). The determination of ionization constants: A laboratory manual. London, Chapman and Hall.
- Čakar M. M., Vasić V. M., Petkovska Lj. T., Stojić D. Lj., Avramov-Ivić M., Milovanović G. A. (1999). Spectrophotometric and electrochemical study of protolytic equilibria of some oximes-acetylcholinesterase reactivators. J. Pharm. Biomed. Anal., 20. 655–662. DOI
- Foretić B., Burger N. (2004). The dissociation constants of 1,1′-bis (pyridinium-4-aldoxime)trimethylene dibromide. Monatshefte für Chemie, 135, 261–267. DOI
- Šinko G., Čalić M., Kovarik Z. (2006). para‐ and ortho‐Pyridinium aldoximes in reaction with acetylthiocholine. FEBS Lett, 580, 3167–3172. DOI
- Worek, F., Reiter, G., Eyer, P. & Szinicz L. (2002). Reactivation kinetics of acetylcholinesterase from different species inhibited by highly toxic organophosphates. Arch. Toxicol., 76, 523–529. DOI
- R Core Team (2018). R: A Language and Environment for Statistical Computing. Available from https://www.R-project.org/.
- Wickham, H. (2009). ggplot2: Elegant graphics for data analysis. N. Y.: Springer-Verlag.
- Elzhov, T. V., Mullen, K. M., Spiess, A.-N. & Bolker B. (2016). minpack.lm: R interface to the Levenberg-Marquardt nonlinear least-squares algorithm found in MINPACK, plus support for bounds. Available from https://CRAN.R-project.org/package=minpack.lm
- Liu, J.-H., Chou, C.-Y., Liu, Y.-L., Liao, P.-Y., Lin, P.-W., Lin, H.-H. & Yang Y.-F. (2008). Acid-base interpretation can be the predictor of outcome among patients with acute organophosphate poisoning before hospitalization. Am. J. Emerg. Med., 26, 24–30. DOI
- Setlur, R. & Sharma, R. M. (2005). Severe metabolic acidosis secondary to organophosphate poisoning. Anesth. Analg., 101, 1894–1898. DOI
- Stefanović D., Antonijević B., Bokonjić D., Stojiljković M. P., Milovanović Z. A., Nedeljković M. (2006). Effect of sodium bicarbonate in rats acutely poisoned with dichlorvos. Basic Clin. Pharmacol. Toxicol., 98, 173–180. DOI
- Clement, J. G. (1993). Recovery from soman-induced hypothermia is due to an increase in acetylcholinesterase activity but not new protein synthesis. Neurotoxicology, 14, 411–416.
- Gordon, C. J. (1996). Thermoregulatory aspects of environmental exposure to anticholinesterase agents. Rev. Environ. Health, 11, 101–117.
- Kamijo, Y., Soma, K., Uchimiya, H., Asari, Y. & Ohwada T. (1999). A case of serious organophosphate poisoning treated by percutaneus cardiopulmonary support. Vet. Hum. Toxicol., 41, 326–328.
- Buckley, N. A., Eddleston, M., Li, Y., Bevan, M. & Robertson J. (2011). Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst. Rev., 16, CD005085. DOI
- Eyer, P. (2003). The role of oximes in the management of organophosphorus pesticide poisoning. Toxicol. Rev., 22, 165–190.