|
SPR Biosensor Based High-Throughput Screening of Low-Weight Compounds Interacting with Candida Krusei Cyp51 1Institute of Biomedical Chemistry, 10 Pogodinskaya str., Moscow, 119121 Russia; *e-mail: leonid.kaluzhskiy@ibmc.msk.ru Key words: surface plasmon resonance (SPR); screening; cytochrome P450 51 (CYP51); low-weight compounds DOI: 10.18097/BMCRM00183 INTRODUCTION
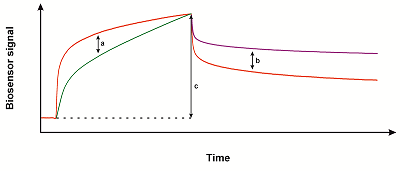
The yeast fungus Candida krusei (C. krusei) is the causative agent of nosocomial infections [1] found primarily in immunocompromised people and in patients with hematological malignancies. C. krusei is naturally resistant to the standard antifungal agent fluconazole [2]. Mortality of fungemia associated with C. krusei is much higher than that with C. albicans [3]. Currently, the drugs of choice for the treatment of fungal infections caused by C. krusei are voriconazole, amphotercin B, and echinocandins [2, 4]. The main target of most antifungal drugs is lanosterol 14-alpha demethylase (cytochrome P450 51, CYP51, EC 1.14.14.154); this enzyme catalyzes one of the key reactions in the ergosterol synthesis pathway [5]. Ergosterol plays an important structural role in the cell membrane of the fungus, therefore, a impairments of its synthesis lead to cell death. The mechanisms of the fungicidal effect of CYP51 inhibitors could be also associated with impairments of various functions of an intact fungal cell, in which CYP51 participates directly or indirectly [5]. However, a number of strains of C. krusei with resistance to azoles have recently been detected in the clinic [2, 4]. In this regard, it seems relevant to search for new non-azole compounds with antifungal activity against C. krusei. One of the possible ways to search for potential drug compounds is the experimental screening of commercially available samples of substances from libraries of compounds of various chemical structures, including collections of naturally occuring compounds. One of the tools for performing such screening can be an optical biosensor based on the effect of surface plasmon resonance (SPR biosensor) [6]. Often such screening is performed using large libraries of small molecules; this increases the likelihood of finding potential candidate compounds. However, the implementation of this approach is associated with the generation of big data; its analysis and interpretation represents a significant difficulty due to the need for manual processing of a huge number of obtained sensorgrams. Biacore Insight Evaluation Software v. 3.0.11.15423, a new generation software for the Biacore 8K SPR biosensor (Cytiva, USA) allows automatic analysis of large amounts of data obtained during the screening of compound libraries. In this paper, we present our protocol for screening a library of natural low molecular weight compounds for interaction with C. krusei CYP51 using the Biacore 8K SPR biosensor. This protocol will be useful for researchers looking for new compounds that can bind to potential target proteins, which is of great importance for the development of new drugs. MATERIALS AND METHODS The recombinant C. krusei CYP51 was used as the target protein (>95% purity according to polyacrylamide gel electrophoresis under denaturing conditions). CYP51 was obtained and purified according to the protocol described previously [7]. The sample of compounds used in the work is part of the collection of natural low molecular weight compounds isolated from marine organisms at the Pacific Institute of Bioorganic Chemistry (Far East Branch of the Russian Academy of Sciences). The following reagents were obtained from the equipment manufacturer (“Cytiva”, USA): HBS-N buffer solution (150 mM NaCl, 10 mM HEPES, pH 7.4), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide-HCl (EDC), N-hydroxysuccinimide (NHS), acetate buffer solutions (10 mM sodium acetate, pH 4.0, 4.5, 5.0, 5.5). Other reagents of analytical grade were obtained from local suppliers. Surface Plasmon Resonance (SPR) SPR analysis was performed using a Biacore 8K optical biosensor (“Cytiva”) with using protocol described previously [8]. The standard optical chips (type CM5, “Cytiva”) coated with carboxymethylated dextran were used. CYP51 was used as a ligand covalently immobilized on the surface of the chip by forming amide bonds between dextran carboxyl groups and free amino groups of the protein. HBS-N was used as a working buffer. All measurements were carried out at 25°C. The results of the analysis presented in the form of sensorgrams, which are a real-time recordings of the biosensor signal in resonance units (RU) (1 RU corresponds to 1 pg of protein on the optical chip surface). The resulting biosensor signal was the signal difference between the working channel with the immobilized ligand protein and the control channel (without the protein). The results of the screening of low molecular weight compounds for interaction with CYP51 were analyzed using a licensed computer program Biacore Insight Evaluation Software v. 3.0.11.15423 (“Cytiva”). pH Optimization of the Immobilization Buffer (pH Scouting) To select the optimal pH value of the immobilization buffer, samples of CYP51 solutions were prepared at a concentration of 20 µg/mL in 10 mM acetate buffer with pH 4.0, 4.5, 5.0, 5.5. The resulting test solutions of CYP51 were sequentially injected for 2 min at a flow rate of 5 µl/min into the liquid system of the biosensor to assess the electrostatic preconcentration of the protein near the surface of the CM5 chip. In this case, the chip surface was not activated with the EDC/NHS mixture. After each injection, the chip surface was washed with 50 mM NaOH for 30 s at a flow rate of 35 μl/min. The preferred choice is a pH value closer to neutral, at which a minimal sufficient level of protein preconcentration is achieved. In our case, the pH 4.5 value was optimal. Covalent Immobilization of CYP51 on the Surface of the CM5 Chip Immobilization of CYP51 on the surface of the CM5 chip was performed using the following procedures: (1) activation of dextran carboxyl groups of the chip by injecting a mixture of equal volumes of 0.2 M EDC and 0.05 M NHS for 7 min at a flow rate of 5 µl/min, (2) injection of a CYP51 solution (20 µg/ml) in immobilization buffer (10 mM acetate buffer, pH 4.5) for 5 min at a flow rate of 5 µl/min, (3) inactivation of the activated carboxyl groups of dextran that did not react with the protein by their hydrolysis while passing the working buffer for 1 h. We excluded the standard protocol of inactivation by injection of 1 M ethanolamine hydrochloride solution recommended by the manufacturer due to exclusion of exposing the immobilized protein to a strong alkaline environment (pH 8.5). Preparation of Small Compound Samples for SPR Screening Stock solutions of small compounds at a concentration of 10 mM were prepared in dimethyl sulfoxide (DMSO). Test solutions of compounds at a final concentration of 50 μM were prepared by diluting stock solutions with HBS-N buffer. The final concentration of DMSO in the analyzed samples was 0.5%. When a sample solution is injected into the measuring system of the biosensor, an abrupt shift of the biosensor signal (the so-called bulk effect) can occur due to the difference in the refractive indices of the sample and the working buffer. This can strongly affect the size and shape of the recorded sensorgram. Therefore, to minimize this effect, we added DMSO to the working buffer to a final concentration of 0.5%. Solutions of tested compound were sequentially injected for 10 min at a flow rate of 10 µl/min. Dissociation was recorded for 5 min. The sensorgrams were recorded at a frequency of 10 Hz. Following the injection of each sample, the working and control channels of the biosensor were washed twice with a regenerating solution (1 M NaCl, 0.25% w/v CHAPS) for 20 s at a flow rate of 30 μl/min. The level of the biosensor signal, at which the screening result was considered as positive, was calculated using the software according to predetermined evaluation parameters. RESULTS AND DISCUSSION Immobilization of a Protein Ligand on the Surface of an Optical Chip For effective covalent immobilization of the ligand protein on the carboxymethylated dextrane surface of an optical chip, it is necessary to create its high local concentration near the surface. This is achieved by electrostatic attraction of positively charged molecules of the target protein to the negatively charged dextran layer on the surface of the optical chip. The carboxymethylated dextran of a standard CM5 chip is negatively charged at pH >3.0. For the formation of a positive charge of protein molecules, it is necessary that the pH of the immobilization buffer should be below the pI of the protein. In addition, the immobilization buffer must have a low ionic strength, since the salt ions shield the charges and thus prevent electrostatic interactions. In preliminary pH scouting experiments (method was described above), it was found that pH 4.5 was sufficient for effective preconcentration of CYP51. For the analysis of protein-protein interactions, an acceptable level of protein-ligand preconcentration with a molecular weight of 50 kDa is 1000-3000 RU. At the same time, in the case of the analysis of interactions of small compounds with an immobilized target protein, the level of its immobilization should be 5000-10000 RU. The method for assessing the level of immobilization and the structure of the sensorgram were described by us earlier [8]. After choosing the optimal pH of the immobilization buffer, one can proceed to the activation of dextran carboxyl groups on the surface of the optical chip. This procedure performed by injecting a mixture of 0.2 M EDC and 0.05 M NHS (1:1 ratio by volume) into the fluid system of the biosensor, converts carboxyl groups of the dextran into active ester succinimide groups. After injection of the activating mixture, an upward shift of the baseline in the sensogram by 100-300 RU is observed (when using CM5 optical chips). An increase in the baseline by more than 300 RU may indicate hyperactivation of the chip surface, which may be undesirable, as it can lead to dextran condensation and a sharp decrease in its charge. This phenomenon leads to a decrease in the protein preconcentration near the chip surface and a decrease in the level of its immobilization. To eliminate the effect of hyperactivation, we recommend to dilute the activating mixture with distilled water two or more times. It may also be useful to reduce the injection time of the activating mixture from 7 min to 3 min. After activation of the chip surface, injection of a solution of the target protein in an immobilization buffer with the optimized pH value is carried out. The duration of injection of the target protein solution through the measuring channel with the activated chip surface usually ranges from 1 min to 15 min. This time should be minimized in order to reduce the exposure of the target protein to a low pH and low ionic strength environment. In this particular example with CYP51, the protein immobilization level was 9600 RU with the injection time of 5 min (Fig. 1). The standard protocol for covalent immobilization of the target protein includes the final inactivation of unreacted dextran active groups by a short injection of 1M ethanolamine hydrochloride, pH 8.5 [9]. This ensures complete elimination of unreacted succinimide groups. However, in the case of a labile ligand protein, there is a possibility of its damage under the action of strongly alkaline solutions. To eliminate this factor, we recommend using solutions of substances containing amino groups, such as glycine or Tris buffer, with a neutral pH value and 1 M concentration. Another way to remove unreacted succinimide groups is to wash the measuring channel with a working buffer for 1 h for their spontaneous hydrolysis with the restoration of the original carboxyl groups. In all cases, complete inactivation of the active groups can be verified by the absence of additional immobilization of the ligand protein upon repeated injection of its solution. Analysis of Small Compound Screening Results for Interaction with CYP51 A set of 31 natural compounds of marine origin (marine echinoderms and plants) was screened for interaction with immobilized C. krusei CYP51 . In this work, the compounds are indicated by a number code. Screening was carried out by sequential injections of 50 µM solutions of compounds through the measuring channel of the biosensor with immobilized protein. For each compound, a sensorgram was obtained with an association phase of 600 s and a dissociation phase of 300 s. Processing of the obtained sensorgrams in Biacore Insight Evaluation Software v. 3.0.11.15423 allowed to analyze the maximum amplitude of the sensorgram, the curvature of the sensorgram (slope), as well as the presence of slow dissociation (slow dissociation) (see Fig. 2). For parameters that evaluate the curvature of the sensorgram and the rate of dissociation, it is possible to set a different degree of sensitivity from low to high. A decrease in sensitivity leads to an increase in the threshold at which the parameter value is evaluated as a positive result (the presence of binding). We performed an analysis of our data for the set of 31 compounds, by varying the sensitivity settings as follows: the maximum sensitivity of slope and slow dissociation (option 1), the minimum sensitivity of slope and slow dissociation (option 3), the minimum sensitivity for slope and the maximum one for slow dissociation (option 3), maximum sensitivity for slope and minimum sensitivity for slow dissociation (option 4). The results are presented in Figure 3. It can be seen that in the case of options 1 and 4 of the sensorgram analysis settings, we were able to detect compounds giving a positive result. In the case of options 2 and 3, such compounds were not observed. Option 2 gives the minimum number of compounds whose interaction with C. krusei CYP51 can be assessed as a positive test result. The fact that compounds 1, 14, 17, and 27 correspond to both criteria simultaneously at settings 1 and 4 and only one criterion at settings 2 and 3 suggests that the slope parameter for these sensorgrams is not significant. These sensorgrams can be characterized by slow dissociation, due to which compounds 1, 14, 17, and 21 can be considered the most preferable, including those with settings 2 and 3. Thus, if we focus on the maximum narrowing of the search circle, minimizing the number of compounds that are selected at the screening stage, these four compounds can be identified as the highest priority, since the most promising drug prototypes are considered to be compounds whose interaction with the target is characterized by slow dissociation [10]. It can be also noted that options 4 and 2 of the analysis parameters settings give the least positive results - 8 compounds out of 31. Options 1 and 3 give 16 compounds each. Thus, we can say that the option 4 of settings seems to be the most preferable for selection of the most promising compounds from a large set. If the task is to approach the assessment more flexibly, we can recommend option 1, which allows to select the most interesting from a larger number of hits, guided by the type of sensorgrams. Thus, the results of this work confirm that multichannel optical SPR biosensors of the Biacore 8K type are quite effective tools for performing high-through screening of small compounds by their interaction with target proteins. New software options for automatic processing of a large number of sensorgrams provide opportunities for flexible selection of lead compounds. To analyze the results of a wide screening, it is preferable to maximize the sensitivity to the curvature of the sensorgram in the association phase and minimize the sensitivity to the dissociation rate. This will allow us to exclude a significant part of the test sample in order to select the most priority compounds. These results may be useful for analyzing the results of screening performed on other models of SPR biosensors that record interactions in real time. COMPLIANCE WITH ETHICAL STANDARDS This article does not contain any research involving humans or using animals as subjects. FUNDING The development of a protocol for high-throughput screening of a library of low molecular weight compounds and optimization of the automated analysis of the obtained sensorgrams was performed within the framework of the Program for Basic Research in the Russian Federation for a long-term period (2021-2030) (№ 122030100168-2). High-throughput screening of a library of low molecular weight compounds of natural origin for their interaction with C. krusei CYP51 was performed within the financial support of RFBR, grant no. 20-04-00014. CONFLICT OF INTERESTS The authors declare no conflict of interests. REFERENCES
|