|
THE INFLUENCE OF ADJUVANTS ON ANTIGENIC PROPERTIES OF PEPTIDE CONSTRUCTS COMPOSED OF HEPATITIS C VIRUSENVELOPE PROTEIN E2 FRAGMENTS
1Institute of Biomedical Chemistry, 8 Pogodinskaya str., Moscow, 119121 Russia; Key words: peptide vaccines, hepatitis C virus, envelope proteins, peptide synthesis, adjuvants, Immunomax DOI: 10.18097/BMCRM00227 INTRODUCTION
Low effectiveness of immune responses to peptide vaccines that have been tried to be introduced into clinical practice (for example, the therapeutic vaccine against hepatitis C [1, 2] and against SARS-CoV2 (EpiVacCorona [3-5]) has not dampened interest in such vaccines. This is due to the advantages of peptide vaccines previously noted in reviews in comparison to vaccines based on whole microorganisms (live and killed), subunit vaccines and vaccines based on recombinant antigen proteins: a) safe peptide production technologies; b) a high degree of standardization of peptide antigens and the absence of foreign genetic material; c) possibility of forming an effective immune response to antigenic determinants that have weak immunogenicity in the whole molecule; d) exclusion of components and fragments of pathogen molecules that cause high reactogenicity, allergic and autoimmune reactions; e) possibility of assembling artificial structures from several different antigenic determinants originating from different antigen molecules [6-9]. However, one of the main disadvantages of peptide vaccines is their inability to stimulate the innate immune system and thereby induce the formation of a sufficiently long-lasting immune response and immunological memory [6-10]. Peptides in the vaccine function as immunogens – molecules to which a specific immune response will be directed, i.e. the formation of antibodies specific to the causative agent of the disease or its main pathogen, the stimulation of cytotoxic and helper T cells. [1, 3, 6-9, 11]. However, an effective formation of immunological memory needs the innate immune system stimulation and a sufficiently long-term effect of the immunogen on cells of the immune system in germinal centers of lymph nodes [6, 8, 9]. Peptides, as a rule, cannot provide this due to the lack of appropriate ligand activity and instability to the action of proteases of biological fluids. Therefore, there is an absolute need for peptide vaccines to select adjuvants and carriers that provide both activation of the innate immune system and increased stability of peptide antigens [8-10]. In this work, we compared the effectiveness of the immune response, i.e. the formation of specific antibodies to peptide antigens composed of highly conserved fragments of the hepatitis C virus (HCV) envelope protein E2, in combination with adjuvants and carriers of various types. METHODS Peptide synthesis Peptides were synthesized by the solid-phase method on a polystyrene divinylbenzene polymer modified with 4-(2',4'-dimethoxyphenyl-aminomethyl)-phenoxymethyl groups (Rink amide) with a capacity of 0.55 mmol of active groups/g polymer (Applied Biosystems, USA) based on the initial resin loading in an amount corresponding to 100 micromoles active groups, or 145.2 mg. α-Amino group-protected with 9-fluorenylmethoxycarbonyl(Fmoc) L-amino acids with the following side-chain functional group protections: Asp(D), Glu(E) – tert-butoxy; Gln(Q), Asn(N), His(H) and Cys(C) – tritile; Arg(R) – 2,2,5,7,8-pentamethylchromane-6-sulfonyl; Lys(K) and Trp(W) – tert-butyloxycarbonyl, Ser(S), Thr(T) and Tyr(Y) – tert-butyl (all from ChemPep, USA) were used. The synthesis was performed on an automatic synthesizer 433A from Applied Biosystems in N,N'-dimethylformamide (Puriss, Spektr-chem, Russia). Fmoc protection was removed from α-amino groups by treatment with 22% solution of 4-methylpiperidine (Acros Organics, Belgium) [11, 12]. Fmoc-amino acids were activated with O-(benzotriazole-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU) and cyanohydroxy aminoacetate ethyl ester (Oxyma Pure; "ChemPep") in the presence of 2,4,6-collidine ("Acros Organics") [13]. A 10-fold excess of Fmoc amino acids was used in the coupling reactions. The peptides were removed from the resin along with the deprotections of the side-chain functional groups by treatment with a mixture of trifluoroacetic acid (TFA):3,6-dioxa-1,8-octandithiol:tri-isopropylsilane:anisole:water 183:5:2:5:5 ( volume/volume) for 2 hours [12, 13]. The peptides were precipitated with a mixture of methyl tert-butyl ether:hexane 1:1 (vol./vol.) and dried over KOH. Peptides were purified by reverse-phase HPLC (ZORBAX SB-C8 column 21 x 250 mm, 7 microns, Agilent 1100 chromatographic station, Agilent, USA), elution by acetonitrile gradient: 0-15 min – from 0 to 32%, 15-45 min – from 32 to 38%, 45-50 min – 38-100% in water in the presence of 0.1% TFA at a rate of 15 ml/min and registration of eluate absorption at 210 nm. The purified peptide preparations were evaporated and lyophilized. The purity of the peptides was determined by analytical HPLC (ZORBAX SB-C8 column 4.6 x 150 mm, 3.5 µm, 100 Å, acetonitrile gradient in 0.1% aqueous TFA solution 0-100% in 50 min, elution rate 1 ml/min) and electrospray ionization mass spectrometry on a high-resolution quadrupole time-of-flight mass spectrometer MaXis 4G (Bruker Daltonics, Germany; registration of positively charged ions in the range of 500-2000 Da). Peptide-containing lipid nanoparticles were prepared via mixing 400 mg squalene («Acros Organics»), 20 mg Lipoid S100 (“Lipoid”, Germany), 400 mg Tween 80 («Ferak Berlin GmbH», Germany), 5 mg peptide in 10.0 ml distilled water with further adjusting pH to 7.2-7.4 by 1M NaOH («Merck», Germany). The mixture was sonicated in an ultrasonic disintegrator Bаndelin Sonopuls HD 2200 («Bandelin», Germany) with a titan rod KE76 at a 70% power, on ice, 6 one-minute cycles. Number of cycles was set in order to achieve a constant light transmission value at 660 nm of at least 60% on an Agilent 8453 spectrophotometer (Agilent, Germany) with HP UV Visible ChemStation version A10.01 software. The resulting emulsion was filtered through a 0.22-µm filter (Merck Millipore, USA). In the filtrate, the particle size was determined by dynamic light scattering using a ZetaSizer Nano ZS analyzer (Malvern, UK) with Malvern ZETASIZER 6.20 software. Peptides not bound to nanoparticles were determined in a 10-fold concentrated ultrafiltrate prepared after ultrafiltration of the resulting preparation using Vivaspin 6 10 kDa (Sigma-Aldrich, USA) by analytical HPLC, as indicated above. Lipid nanoparticles without peptides were obtained in a similar way. Peptide conjugates with Immunomax (IM) were prepared with the use of “Immunomax” preparation kindly donated by “Immapharma” (Russia) and additionally purified by dialysis against water and by gel-filtration on TSK-75 column (“Toyo Soda”, Japan, 950x30 mm) eluted with water. Freeze-dried high-molecular fraction, which was eluted immediately after the free volume of the column, was used for conjugate preparations. Conjugation was performed using diethyl ether of squaric acid (3,4-diethoxy-3-cyclobutene-1,2-dione; DESA; Acros organics) in two stages [14]. At the first stage, IM was activated by DEQA in 0.1 M Na-phosphate buffer, pH 7.15 at a mass ratio of IM:DESA 1:1.7, activated IM was separated from the reagent by gel filtration (see above) and lyophilized. Peptides CR4-CR5 and CR5-CR3 (Table 1) were dissolved in a mixture of 8 M urea aqueous solution and dimethyl sulfoxide (1:4 by volume) to a concentration of 1.0 mg/ml. 0.5 ml of the peptide solution was added to 0.5 ml of the solution of IM modified with DESA (1.5 mg) in 0.1 M phosphate buffer, pH 7.45, after which 50 µl 0.5M of Na-borate buffer, pH 8.5 was added and the mixture was left for 12 hours at 4 ° C. The conjugates were dialyzed sequentially against 8 M and 4 M urea solutions and 0.1 M phosphate buffer, pH 7.45. The conjugate solutions obtained were intensively opalescent and contained a small amount of sediment. For administrations to mice, conjugate solutions were diluted with 0.9% NaCl solution “for injections” to the required concentration of 5 µg, 20 µg and 50 µg of conjugate (per IM) in 100 µl of solution. Peptide and conjugate immunogenicities were determined as specific antibody titers in sera of mice immunized with corresponding preparations. A group of 6 BALB/c mice, including 3 males and 3 females, were immunized with each preparation. The preparations (Table 2) were subcutaneously injected in a volume of 100 µl at 6 points on the back three times with an interval of two weeks. For immunization, peptides were dissolved in 0.85% NaCl solution with 10 mM Na-phosphate buffer pH 7.2 (phosphate buffered saline; PBS) to a peptide concentration of 1 mg/ml. Samples with Freund's adjuvant (1st immunization with full adjuvant, 2nd and 3rd with incomplete adjuvant) were obtained by mixing 1 volume of a peptide solution with 1 volume of adjuvant. Samples of peptides in the form of a mechanical mixture with IM were obtained by mixing 1 volume of a peptide solution and 1 volume of the purified IM solution at a concentration of 1.5 mg/ml. Mice were decapitated a week after the last injection, and serum was obtained from the collected blood, aliquoted and stored at -20 °C until antibody titers were determined. Determination of antibody titers against HCV peptides and envelope proteins E2 and E1E2 was achieved by enzyme immunoassay as previously described [15, 16], except that peptides CR4-CR5 and CR5-CR3 were adsorbed onto the surface of wells of immunological plates with high adsorption capacity directly from 0.1 M Na-bicarbonate buffer, pH 8.5. E2 protein (water-soluble form and E1E2 heterodimer) were kindly provided by Dr. J. Dubuisson, Institute of Biology, Lille, France. In silico search for T-helper epitopes in highly conserved fragments of the protein E1 and E2 amino acid sequences was carried out using software products from the SYFPEITHI [17] and IEDB [18] databases. RESULTS AND DISCUSSION Previously, we proposed an approach to the design of peptide immunogens for the hepatitis C vaccine, which consisted in combining highly conserved B and T helper epitopes from envelope proteins E1 and E2 in one artificial peptide construct, and the selected B epitope should have functional activity essential for HCV [19]. We confirmed the effectiveness of this approach in terms of the formation of antibodies specific to both B-epitopes and whole envelope proteins of the virus [19]. The immunogenicity of such constructs with the formation of both peptide and protein-specific antibodies was demonstrated only when administered with Freund's adjuvant (AF), which indicates an insufficiently effective stimulation of the immune response by peptides alone and their incorrect orientation in the aggregates/micelles formed in the solution [19]. However, AF cannot be used in human vaccines because of its high reactogenicity [10]. Therefore, we tested the immunogenicity of peptide constructs from highly conserved fragments of HCV E2 envelope protein in the form of preparations with pharmaceutically acceptable adjuvants and carriers. The peptide constructs used in the experiment (Table. 1) are composed of the highly conserved regions CR3, CR4 and CR5 (designations according to [20]) of the E2 protein.
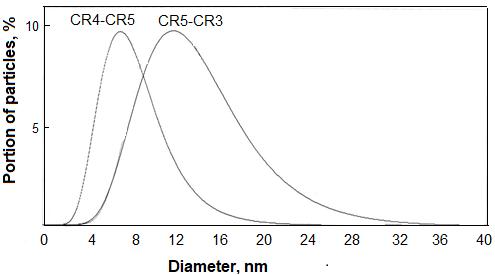
CR3 and CR4 bound heparan sulfates responsible for the primary fixation of the virus on cells [21], Respectively, antibodies against these B epitopes could block the primary fixation of the virus on cells [22, 23]. CR5 contains T helper epitopes identified in silico using SYFPEITHI [17] and IEDB [18] software products and confirmed in experiments on animal immunization [19] and activation of T-lymphocytes in patients with hepatitis C [24]. In addition, antibodies specific to at least two linear B epitopes of the CR5 region were detected in patients with hepatitis C [16]. In order to determine the effect of the adjuvant and carrier on the immunogenicity of the obtained peptide constructs, mice were immunized with the following preparations: 1) peptides dissolved in phosphate-salt buffer, 2) mixtures of these solutions with AF, 3) peptides incorporated in phospholipid-squalene nanoparticles (PSN), 4) conjugates and 5) mixtures of IM with peptides, 6) mixtures of peptide-IM conjugates with PSN and 7) conjugates of peptides with dextran. The use of carrier-free peptides for immunization is justified by the fact that these peptide constructs formed particles with a diameter of 3-12 nm (median 5 nm; CR4-CR5) and 5-28 nm (median 12 nm; CR5-CR3), which indicated oligomerization in solutions with a concentration of 1 mg/ml (Fig. 1).
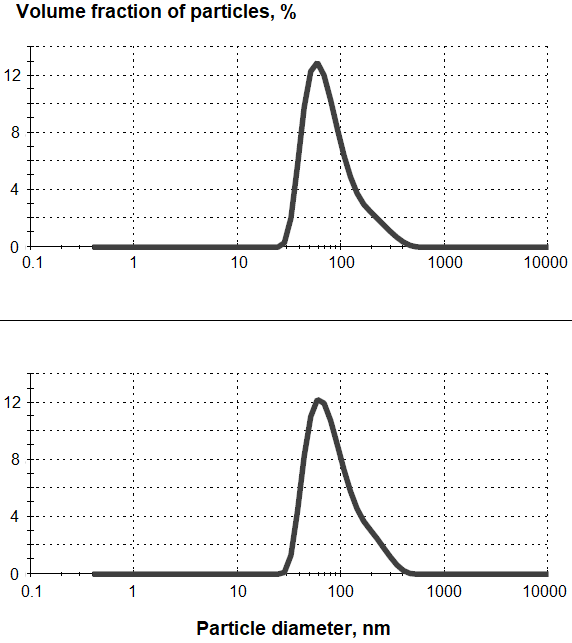
In some cases, the immunogenicity of such micelles was noted in animals immunized with them, accompanied by the formation of antipeptide antibodies [19]. Squalene is used as an immunostimulator in the composition of emulsion adjuvants in modern vaccines [1-3, 10, 25]. Our attempt to use PSN to increase the immunogenicity of peptide constructs was inspired by the effectiveness of PSN stabilized with the pharmaceutically acceptable detergent Tween 80 as both a carrier and an adjuvant for subunit vaccines [26]. Peptides were included in the composition of PSN with Tween 80 during the preparation by ultrasound treatment. The obtained PSN preparations with peptides contained nanoparticles with an average diameter of 89-99 nm. (Fig. 2).
After ultrafiltration of PSN with peptides the peptides were detected in a concentrated filtrate by HPLC with mass detection in trace amounts, which indicated their rather strong adsorption on PSN or incorporation into PSN. IM, a high–molecular-weight lipoproteoglycan from potato sprouts [27], capable of activating cells of the immune system responsible for innate immunity through toll-like type 4 receptors and registered as a drug with immunomodulatory activity, was another carrier of peptide antigens with adjuvant activity tested by us [27, 28]. Previously, IM was successfully used as a carrier in the production of monoclonal antibodies against non-structural HCV proteins [29]. Since IM contains free amino groups, peptide constructs also containing free amino groups were conjugated with this proteoglycan using DESA, two carboxy groups of which are activated at different pH values and provide sequential crosslinking of two amino-containing compounds [14]. Since IM contains almost no absorbing components at 280 nm, the conjugation of peptides could be estimated by increasing the optical density of the conjugate solution at this wavelength, however, the strong opalescence of the conjugate solution did not allow an accurate assessment of the amount of bound peptide. The complex composition and high molecular weight of the IM components also made it impossible to estimate the amount of the peptide conjugated with it using amino acid analysis or by changing the molecular weight. According to previously published data on the number of moles of conjugated peptides per unit mass of IM [29], we assumed an approximate content of conjugated peptides in the conjugate preparation of 1% of the IM mass. In addition to peptide conjugates with IM, we tested the immunogenicity of a mechanical mixture of peptide constructs with IM, in which proteoglycan acted only as an adjuvant, as well as a peptide conjugate with dextran, to study the effect of a high molecular weight, but not an immunoreactive carrier. The effectiveness and specificity of the immune response to the administration of the above-mentioned peptide preparations to groups of mice consisting of an equal number of males and females was evaluated. Specificity was assessed by the formation of antibodies both against individual fragments of peptide constructs as B-epitopes, and against the whole envelope protein E2 and the HCV E1E2 heterodimer. Table 2 shows the results of determining the titers of anti-peptide and anti-protein antibodies in animals immunized with peptide preparations in composition or as conjugates with various adjuvants. As can be seen from the table, the peptides CR4-CR5 and CR5-CR3 differ greatly in immunogenicity: the peptide CR5-CR3 caused the formation of specific antibodies in mice even when administered without adjuvants, whereas to obtain antibodies against the peptide CR4-CR5, even the simple addition of an adjuvant was not enough, and conjugation of this peptide with IM carrier with adjuvant properties was required. In the case of CR4-CR5 peptide, both the fixation of the antigen on a high-molecular carrier and the stimulation of the immune system towards the formation of specific antibodies were apparently important. The conjugate of this peptide with IM caused the formation of antibodies, binding to the native HCV–E2 envelope proteins and the E1E2 heterodimer, which may indicate the formation of a CR4-CR5 peptide structure in the conjugate similar to that in the envelope protein. However, this structure possibly does not represent a linear region, since no antibodies against individual parts of the peptide (CR4 and CR5) have been detected in the blood sera of immune animals.
CR5-CD3 peptide immunogenicity was also significantly increased after the conjugation with IM: a higher antibody titer was achieved with a 100-fold lower dose of the peptide. It is interesting to note that when this peptide was injected together with AF, the formation of protein-specific antibodies was not noted, unlike in the case of the free peptide; AF probably prevented the formation of the peptide spatial structure similar to that in the whole protein. Antibodies against the CR3 fragment at titers 1:500-1:1000 were detected in the blood sera of mice immunized with the IM-CR5-CD3 conjugate; no antibodies against the CR5 fragment were detected. PSN as an adjuvant turned out to be less effective than IM. However, the addition of P SN to the IM-CR5-CD3 conjugate slightly increased the effectiveness of the formation of specific antibodies. CONCLUSIONS IM as a carrier with adjuvant properties proved to be the best among the studied adjuvants for peptide immunogenic constructs composed of highly conserved fragments of the HCV E2 envelope protein. It is unclear what is the difference in the mechanism of presentation of antigens associated with IM and PSN, since the fine mechanisms of stimulating the immune response with squalene have not yet been investigated [25]. With regard to IM, it can be assumed that its activation of dendritic cells through TLR4 activates the mechanisms of innate immunity, which are subsequently involved in the formation of adaptive immunity, including the formation of specific antibodies [27-30]. In addition, conjugation of peptides with IM can contribute to their stabilization, increase entry into antigen-presenting cells, and create a pool of slowly released immunogen at the injection site. IM should probably be considered as one of the most effective adjuvants for peptide vaccines. COMPLIANCE WITH ETHICAL STANDARDS The research on laboratory animals was approved by the IBMH Ethics Commission, Protocol No. 2 from 12.02.2015. ACKNOWLEDGEMENTS The authors are grateful to Dr. J. Dubuisson (Institut biologie de Lille, France) for providing with E2 protein and E1E2 heterodimer preparations, to A.V. Talanova and Yu.Yu. Khudoklinova (IBMC) for their assistance with animal research, and also to Immafarma LLC for donating the Immunomax preparation. Synthesis and mass spectrometric analysis of peptides were performed using the equipment of the IBMC Core Facility “Human Proteome" FUNDING Peptide CR5-CR3 structure development was performed in the framework of the State Contract No. 14N08.12.0025 from 08.08.2013 with the Ministry of Education and Science. Pilot experiments were carried out within the framework of the Program of Fundamental Scientific Research of the State Academies of Sciences for 2013-2020 (theme No. 0518-2014-0003), the work was completed within the framework of the Program of Fundamental Scientific Research of the State Academies of Sciences for 2021-2030 (theme No. 122030100170-5). CONFLICT OF INTERESTS R.I. Ataullakhanov and T.M. Melnikova are the authors of the RF Patent 2,563,818 dated 11/21/2013, describing the method of preparation of IM and its immunostimulating activity [27]. E.A. Egorova and E.F. Kolesanova are the authors of the RF Patent 2,675,108 dated 06/15/2015. "A composition based on synthetic peptides and lipids for the hepatitis C vaccine", describing an immunogenic composition that includes the peptide CR5-CR3; the patent holder is IBMC. The other authors declare no conflict of interests. Preliminary results of the work on testing the immunogenicity of peptide conjugates CR4-CR5, CR5-CR3 with IM in comparison with peptide compositions with 20-nm phospholipid nanoparticles were presented as poster presentations at the forum "Days of Immunology in St. Petersburg" in 2015 and at the 36th European Peptide Symposium in Leipzig (Germany) in 2016. REFERENCES
|