L-Asparaginase Conjugates of Rhodospirillum Rubrum with Polymers of Different Structures with Improved Biocatalytic Properties
1Moscow State University, 1 Leninskie Gory str., Moscow, 119991 Russia
2Institute of Biomedical Chemistry, 10 Pogodinskaya str., Moscow, 119121 Russia; *e-mail: natdobryak@gmail.com
Keywords: L-asparaginase; Rhodospirillum rubrum; covalent conjugates; CD spectrometry; IR spectrometry; polymers
DOI:10.18097/BMCRM00229
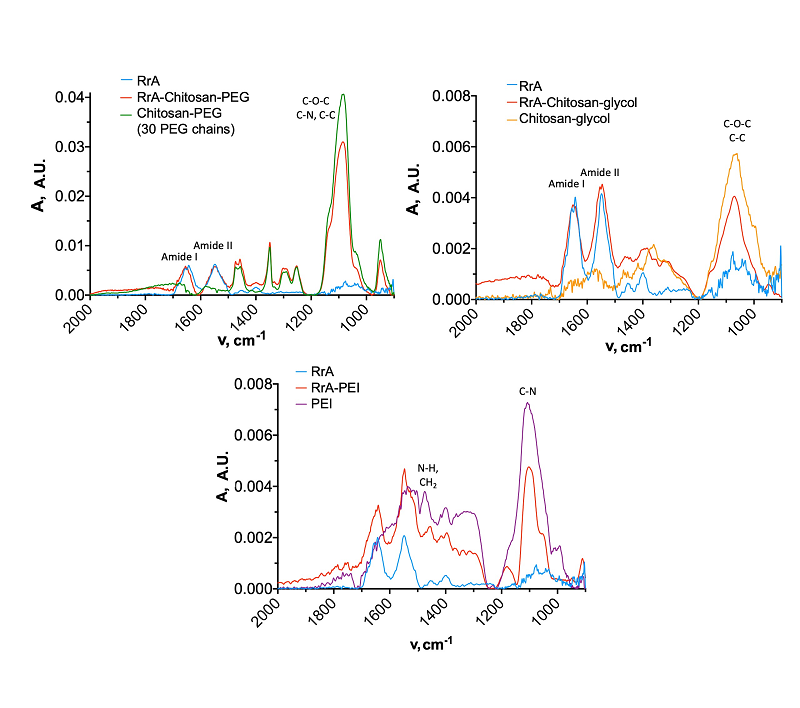
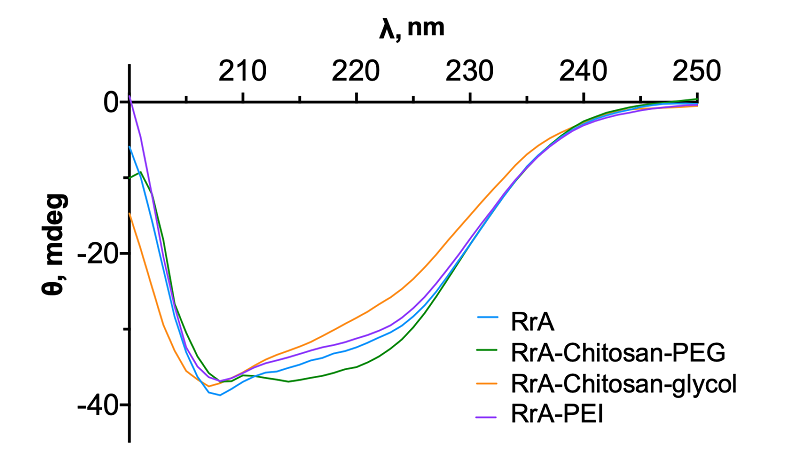
L-asparaginase from the mesophyllic bacterium Rhodospirillum rubrum shows promise as a potential biomedicine treatment for tumor diseases. To enhance the enzyme’s catalytic parameters, we have synthesized covalent conjugates with chitosan-PEG, chitosan-glycol and polyethylenimine. Interestingly, binding to these polymers had minimal impact on the enzyme’s substrate binding constant. The RrA-PEI conjugate showed the most significant changes in secondary structure content. The success of the previous modification was evaluated by IR spectroscopy, which indicated that the polycations used in the work have a positive effect on the catalytic activity of the enzyme. The RrA-chitosan-PEG conjugate demonstrated the highest enzymatic activity.
|
Close

|
Table 1.
Characteristics of polymers used in the work.
|
|
Close

|
Table 2.
Percentages of secondary structure elements of the native enzyme and its conjugates,
as counted in the CDNN program. |
|
Close

|
Table 3.
Catalytic parameters and zeta potential values of native RrA and its conjugates. The hydrolysis activity of 20 mM L-Asn was measured in 10 mM PBS pH 7.5 at 37°C
|
FUNDING
The work was done in the framework of the Russian Federation fundamental research program for the long-term period (2021-2030) (No. 122022800499-5). The work was performed using equipment (Bruker Tensor 27 FT-IR spectrometer (Germany), MICRAN-3 FTIR microscope (Simex, Russia), and Jasco J-815 circular dichroism spectrometer (Jasco, Japan)) of the Lomonosov Moscow State University Development Program.
REFERENCES
- Sokolov, N.N., Eldarov, M.A., Pokrovskaya, M.V., Aleksandrova, S.S., Abakumova, O.Y., Podobed, O.V., Melik-Nubarov, N.S., Kudryashova, E.V., Grishin, D.V., Archakov, A.I. (2015) Bacterial recombinant L-asparaginases: Properties, structure and anti-proliferative activity. Biomeditsinskaya Khimiya, 61(3), 312–324. DOI
- Piątkowsk-Jakubas, B., Krawczyk-Kuliś, M., Giebel, S., Adamczyk-Cioch, M., Czyż, A., Lech-Marańda, E., Paluszewska, M., Pałynyczko, G., Piszcz, J., Hołowiecki, J. (2008) Use of L-asparaginase in acute lymphoblastic leukemia: Recommendations of the Polish adult leukemia group. Polish Archives Internal Medicine, 118(11), 664–669. DOI
- Fernandez, C.A., Stewart, E., Panetta, J.C., Wilkinson, M.R., Morrison, A.R., Finkelman, F.D., Sandlund, J.T., Pui, C.H., Jeha, S., Relling, M.V., Campbell, P.K. (2014) Successful challenges using native E. coli asparaginase after hypersensitivity reactions to PEGylated E. coli asparaginase. Cancer Chemother. Pharmacol., 73(6), 1307–1313. DOI
- Pokrovskaya, M.V., Pokrovskiy, V.S., Aleksandrova, S.S., Anisimova, N.Yu., Andrianov, R.M., Treschalina, E.M., Ponomarev, G.V., Sokolov, N.N. (2012) Recombinant intracellular Rhodospirillum rubrum L-asparaginase with low L-glutaminase activity and antiproliferative effect. Biomeditsinskaya Khimiya, 59(2), 192–208. DOI
- Malakhova, M.A., Pokrovskaya, M.V., Alexandrova, S.S., Sokolov, N.N., Kudryashova, E.V. (2018) Regulation of catalytic activity of recombinant L-asparaginase from Rhodospirillum rubrum by conjugation with a PEG-chitosan copolymer. Moscow University Chemistry Bulletin, 73, 185–191. DOI
- Deygen, I.M., Kudryashova, E.V. (2014) Structure and stability of anionic liposomes complexes with PEG-chitosan branched copolymer. Russ. J. Bioorg. Chem., 40, 547–557. DOI
- Sukhoverkov, K.V., Kudryashova, E.V. (2015) PEG-chitosan and glycol-chitosan for improvement of biopharmaceutical properties of recombinant L-asparaginase from Erwinia carotovora. Biochemistry (Moscow), 80, 113–119. DOI
- Deygen, I.M., Kudryashova, Е.V. (2016) New versatile approach for analysis of PEG content in conjugates and complexes with biomacromolecules based on FTIR spectroscopy. Colloids Surfaces B: Biointerfaces, 141, 36–43. DOI
- Kudryashova, E.V., Visser, A.J.W.G., Jongh, H.H.J.D.E. (2005) Reversible self-association of ovalbumin at air-water interfaces and the consequences for the exerted surface pressure. Protein Sci., 14, 483–493. DOI
- Kudryashova, E.V., Suhoverkov, K.V., Sokolov, N.N. (2015) PEG-chitosan branched copolymers to improve the biocatalytic properties of Erwinia carotovora recombinant L-asparaginase. Biomeditsinskaya Khimiya, 61(4), 480–487. DOI
- Kuchumova, A.V., Krasotkina, Y.V., Khasigov, P.Z., Sokolov, N.N. (2007) Modification of recombinant asparaginase from Erwinia carotovora with polyethylene glycol 5000. Biomeditsinskaya Khimiya, 53(1), 107–111.
- Ramirez-Paz, J., Saxena, M., Delinois, L.J., Joaquin-Ovalle, F.M., Lin, S., Chen, Z., Rojas-Nieves, V.A., Griebenow, K. (2018) Thiol-maleimide poly(ethylene glycol) crosslinking of L-asparaginase subunits at recombinant cysteine residues introduced by mutagenesis. PLoS ONE, 13(7), e0197643. DOI