NOVEL PHOSPHONIUM SALT DERIVATIVES INDUCE DNA DAMAGE AND APOPTOSIS IN CERVICAL CANCER CELLS
Arbuzov Institute of Organic and Physical Chemistry, FRC Kazan Scientific Center, Russian Academy of Sciences, 8 Arbuzov str., Kazan, 420088 Russia; *e-mail: aplyubina@gmail.com
Keywords: phosphonium salts; apoptosis; DNA damage; cell cycle
DOI:10.18097/BMCRM00254
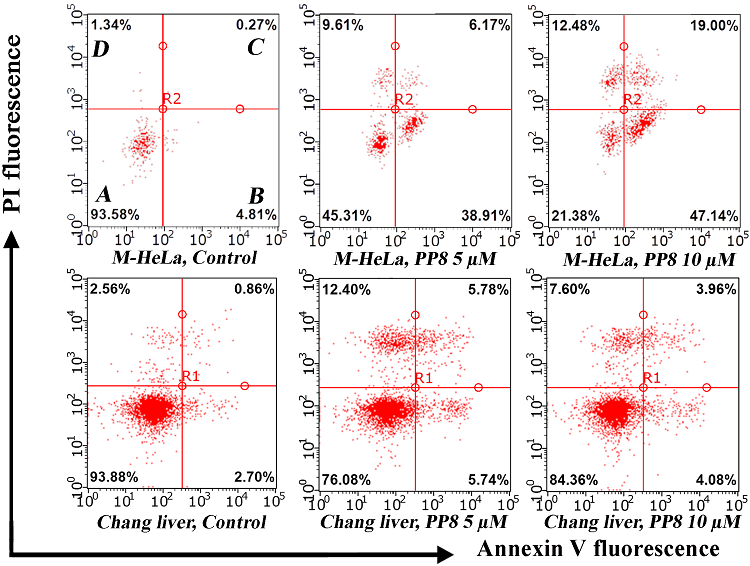
This study demonstrates that (Z)-(2-(2-hydroxy-5-chlorophenyl)-2-phenylethenyl)alkyldiphenylphosphonium chlorides exhibit high antitumour activity, comparable to that of the reference drug doxorubicin. The phosphonium salts exhibited reduced toxicity towards conditionally normal cell lines in the majority of cases. The mechanism of action of compound PP8, which contains an octyl radical at the phosphorus atom, on the model cell line M-HeLa included partial cell cycle arrest in the G1 phase, an increased generation of reactive oxygen species, and an induction of mitochondrial apoptosis. These experimental data are supported by the elevated levels of p53, p21, H2A.X and caspase-9 proteins detected by multiplex analysis. The results indicated the occurrence of double-strand DNA breaks under the influence of the studied compound. Therefore, additional structural modifications to enhance the selectivity of the studied compounds will provide a basis for the development of novel effective antitumour agents.

|
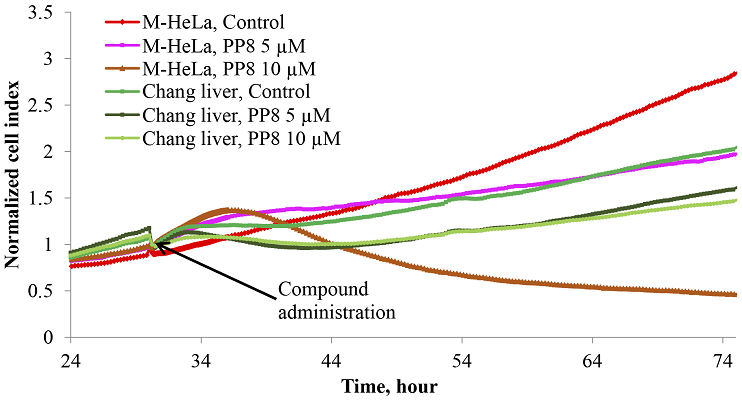
Figure 1.
Changes in the adhesive properties of M-HeLa and Chang liver cells in a real-time experiment when exposed to different concentrations of the PP8 compound.
|

|
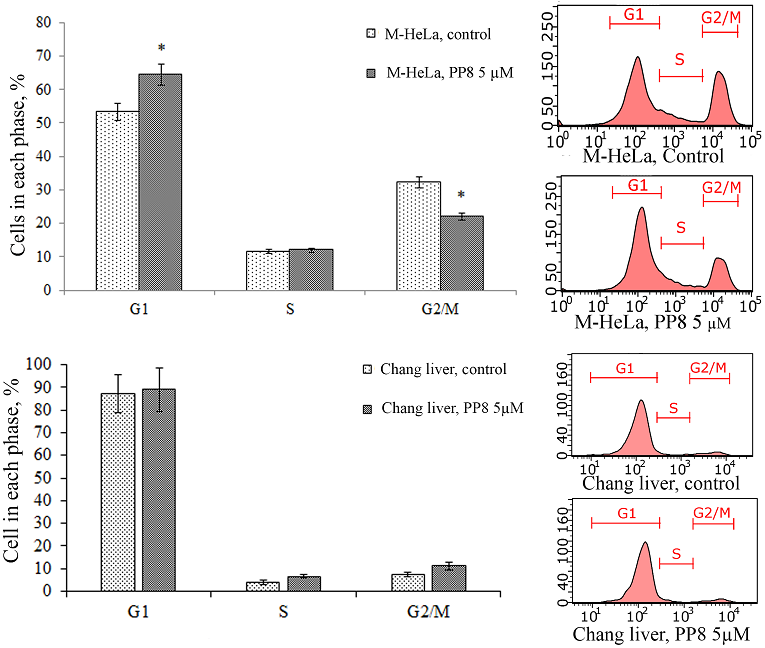
Figure 2.
Effect of compound PP8 on the cell cycle of M-HeLa and Chang liver cells.* - p<0.01 when compared with the control sample.
|

|
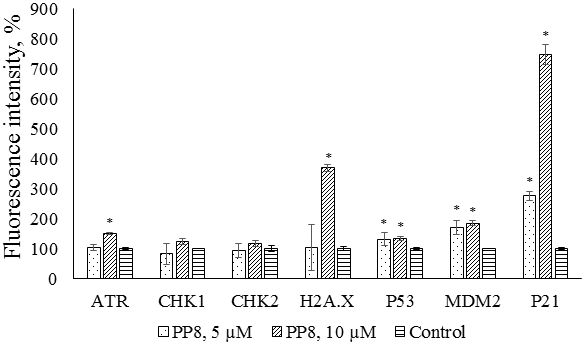
Figure 3.
Multiplex analysis of DNA damage/genotoxicity markers in M-HeLa cells after exposure to compound PP8. * - p<0.01 when compared with the control sample.
|

|
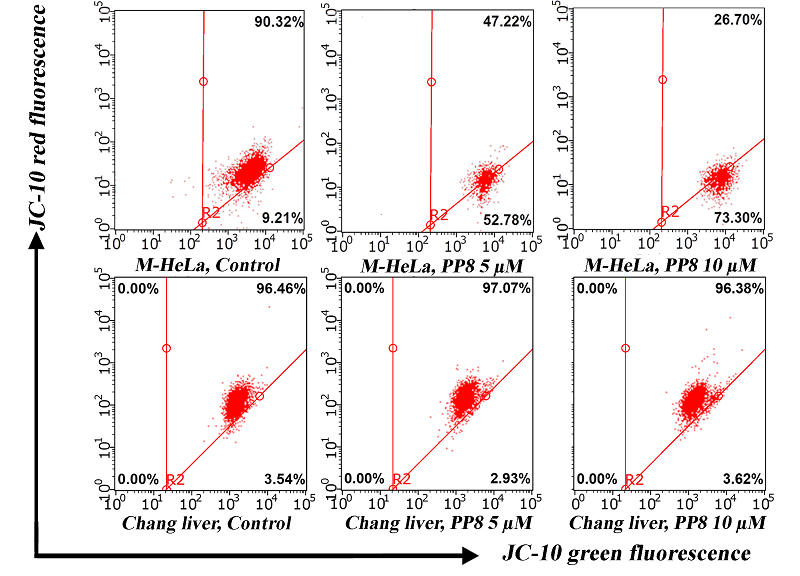
Figure 5.
Changes in mitochondrial membrane potential in M-HeLa and Chang liver in the presence of compound PP8.
|

|
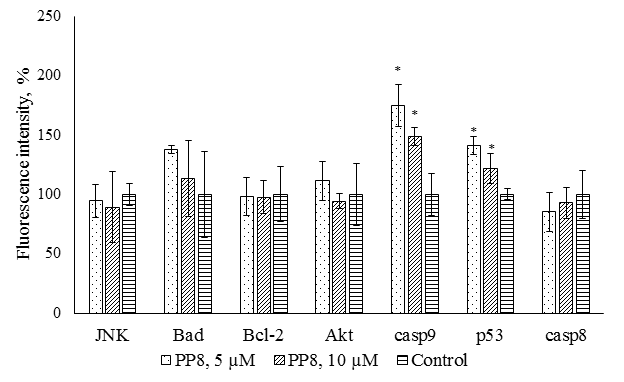
Figure 6.
Multiplex analysis of early apoptosis markers in M-HeLa cells after exposure to compound PP8. * - p<0.01 when compared with the control sample.
|

|
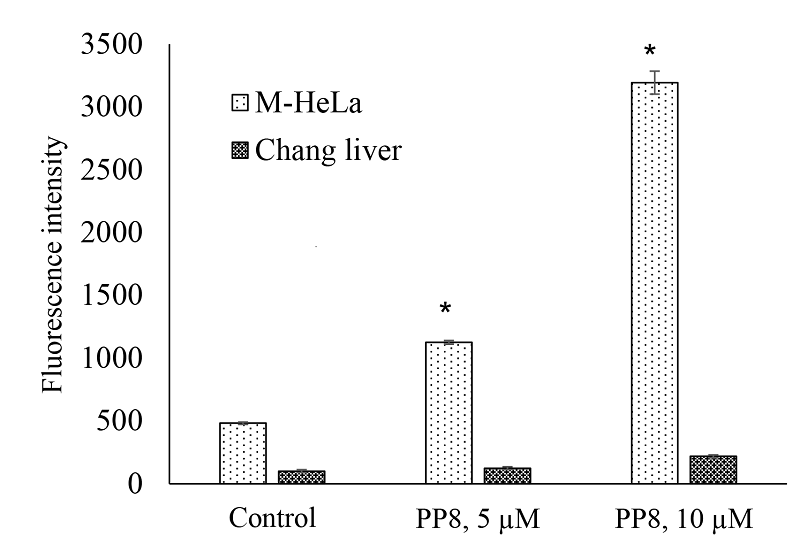
Figure 7.
ROS level in M-HeLa and Chang liver cells in the presence of compound PP8; * - p<0.01 when compared with the control sample.
|
|
CLOSE

|
Table 1.
Cytotoxicity of the studied compounds against human cell lines of various origins.
|
|
CLOSE

|
Table 2.
Selectivity index (SI) of the studied compounds, calculated in relation to IC50 in conditionally normal Chang liver and RPMI 1788 cells.
|
FUNDING
The work was carried out within the framework of the state assignment of the FRC Kazan Scientific Center of RAS.
REFERENCES
- Makimbetov, E.K., Salikhar, R.I., Tumanbaev, A.M., Toktanalieva, A.N.,Kerimov, A.D. (2020) Cancer epidemiology in the world. Modern problems ofscience and education, 2, 168-168. DOI
- Global cancer burden growing, amidst mounting need for services. RetrievedSeptember 30, 2024, from: https://www.who.int/news/item/01-02-2024-globalcancer-burden-growing--amidst-mounting-need-for-services
- Kaprin, A.D., Starinsky, V.V., Shakhzadova, A.O. (2022). Malignantneoplasms in Russia in 2021 (incidence and mortality). Moscow: PA HertsenMoscow Oncology Research Institute–Branch of the National Medical ResearchRadiological Center. 252 p. ISBN 978-5-85502-280-3
- Peyraga, G., Ducassou, A., Arnaud, F.X., Lizée, T., Pouédras, J., Moyal, É.(2021). Radiothérapie et toxicité médullaire: actualités et perspectives. Cancer/Radiothérapie, 25(1), 55-61. DOI
- Zhu, S., Wang, X., Jiang, H. (2024). Systematic Reversal of Drug Resistancein Cancer. Targets, 2(3), 250-286. DOI
- Pawar, A., Korake, S., Pawar, A., Kamble, R. (2023). Delocalized lipophiliccation triphenyl phosphonium: promising molecule for mitochondria targeting.Current Drug Delivery, 20(9), 1217-1223. DOI
- Ibrahim, M.K., Haria, A., Mehta, N.V., Degani, M.S. (2023). Antimicrobialpotential of quaternary phosphonium salt compounds: A review. FutureMedicinal Chemistry, 15(22), 2113-2141. DOI
- Nissim, M., Lline-Vul, T., Shoshani, S., Jacobi, G., Malka, E., Dombrovsky,A., Banin, E., Margel, S. (2023). Synthesis and Characterization of DurableAntibiofilm and Antiviral Silane-Phosphonium Thin Coatings for Medical andAgricultural Applications. ACS omega, 8(42), 39354-39365. DOI
- Tatarinov, D.A., Kuznetsov, D.M., Voloshina, A.D., Lyubina, A.P., Strobykina,A.S., Mukhitova, F.K., Polyancev, F.M., Mironov, V.F. (2016). Synthesis of2-(2-hydroxyaryl) alkenylphosphonium salts from phosphine oxides via ringclosingring-opening approach and their antimicrobial evaluation. Tetrahedron,72(51), 8493-8501. DOI
- Terekhova, N.V., Lyubina, A.P., Voloshina, A.D., Sapunova, A.S., Khayarov,K.R., Islamov, D.R., Usachev, K.S., Evtugyn, V.G., Tatarinov, D.A., Mironov,V.F. (2022). Synthesis, biological evaluation and structure-activity relationshipof 2-(2-hydroxyaryl) alkenylphosphonium salts with potency as anti-MRSAagents. Bioorganic Chemistry, 127, 106030. DOI
- Rokitskaya, T.I., Terekhova, N.V., Khailova, L.S., Kotova, E.A., Plotnikov,E.Y., Zorov, D.B., Tatarinov, D.A., Antonenko, Y.N. (2019). Zwitterionicprotonophore derived from 2-(2-hydroxyaryl)alkenylphosphonium as anuncoupler of oxidative phosphorylation. Bioconjugate Chemistry, 30(9), 2435-2443. DOI
- IC50 Calculator. Retrieved June 11, 2024, from: https://www.aatbio.com/tools/ic50-calculator
- Kho, D., MacDonald, C., Johnson, R., Unsworth, C.P., O’Carroll, S.J.,Du Mez, E., Angel, C.E., Graham, E.S. (2015). Application of xCELLigenceRTCA biosensor technology for revealing the profile and window of drugresponsiveness in real time. Biosensors, 5(2), 199-222. DOI
- Terekhova, N.V., Tatarinov, D.A., Shaihutdinova, Z.M., Pashirova, T.N.,Lyubina, A.P., Voloshina, A.D., Sapunova, A.S., Zakharova, L.Ya., Mironov, V.F.(2020). Design and synthesis of amphiphilic 2-hydroxybenzylphosphoniumsalts with antimicrobial and antitumor dual action. Bioorganic & MedicinalChemistry Letters, 30(13), 127234. DOI
- Chen, Z., Luo, R., Xu, T., Wang, L., Deng, S., Wu, J., Wang, H., Lin, Y.,Bu, M. (2024). Design, synthesis and antitumor effects of lupeol quaternaryphosphonium salt derivatives. Bioorganic & Medicinal Chemistry, 113, 117934. DOI
- Abassi, Y.A., Xi, B., Zhang, W., Ye, P., Kirstein, S.L., Gaylord, M.R.,Feinstein, S.C., Wang, X., Xu, X. (2009). Kinetic cell-based morphologicalscreening: prediction of mechanism of compound action and off-target effects.Chemistry & biology, 16(7), 712-723. DOI
- Xie, W., Ye, Y., Shen, A., Zhou, L., Lou, Z., Wang, X., & Hu, J. (2008).Evaluation of DNA-targeted anti-cancer drugs by Raman spectroscopy.Vibrational Spectroscopy, 47(2), 119–123. DOI
- Bohgaki, T., Bohgaki, M., Hakem, R. (2010). DNA double-strand breaksignaling and human disorders. Genome integrity, 1, 1-14. DOI
- Ramazanov, B.R., Khusnutdinov, R.R., Galembikova, A.R., Dunaev, P.D.,Boichuk S.V. Role of p53 protein in activation of ATM- and PARP-mediatedDNA damage repair (DDR) pathways induced by topoisomerase type IIinhibitors. Kazan medical journal, 97(2), 245-249. DOI
- Smith, J., Tho, L.M., Xu, N., Gillespie, D.A. (2010). The ATM–Chk2 andATR–Chk1 pathways in DNA damage signaling and cancer. Advances in cancerresearch, 108, 73-112. DOI
- Abbas, I., Badran, G., Verdin, A., Ledoux, F., Roumie, M., Guidice, J.M.L.,Courcot, D., Garçon, G. (2019). In vitro evaluation of organic extractablematter from ambient PM2. 5 using human bronchial epithelial BEAS-2B cells:Cytotoxicity, oxidative stress, pro-inflammatory response, genotoxicity, and cellcycle deregulation. Environmental research, 171, 510-522. DOI
- Ismail, I.H., Hendzel, M.J. (2008). The γ‐H2A. X: Is it just a surrogatemarker of double‐strand breaks or much more?. Environmental and molecularmutagenesis, 49(1), 73-82. DOI
- Craig, A., Scott, M., Burch, L., Smith, G., Ball, K., Hupp, T. (2003).Allosteric effects mediate CHK2 phosphorylation of the p53 transactivationdomain. EMBO reports, 4(8), 787-792. DOI
- Loughery, J., Cox, M., Smith, L.M., Meek, D.W. (2014). Critical role forp53-serine 15 phosphorylation in stimulating transactivation at p53-responsivepromoters. Nucleic acids research, 42(12), 7666-7680. DOI
- Liebl, M.C., Hofmann, T.G. (2019). Cell fate regulation upon DNA damage:p53 serine 46 kinases pave the cell death road. Bioessays, 41(12), 1900127. DOI
- Karimian, A., Ahmadi, Y., Yousefi, B. (2016). Multiple functions of p21 incell cycle, apoptosis and transcriptional regulation after DNA damage. DNArepair, 42, 63-71. DOI
- Liebl, M.C., Hofmann, T.G. (2019). Cell fate regulation upon DNA damage:p53 serine 46 kinases pave the cell death road. Bioessays, 41(12), 1900127. DOI
- Reczek, C.R., Chandel, N.S. (2017). The two faces of reactive oxygenspecies in cancer. Annual review of cancer biology, 1(1), 79-98. DOI
- Sui, X., Wang, J., Zhao, Z., Liu, B., Liu, M., Liu, M., Shi, C., Feng X., Fu Y.,Shi D., Li S., Qi Q., Xian Mo., Zhao, G. (2024). Phenolic compounds induceferroptosis-like death by promoting hydroxyl radical generation in the Fentonreaction. Communications Biology, 7(1), 199. DOI
- Wang, Q.Y., Xu, Y.S., Zhang, N.X., Dong, Z.P., Zhao, B.N., Liu, L.C., Lu T.,Wang, Y. (2020). Phenylboronic ester-modified anionic micelles for ROS-stimuliresponse in HeLa cell. Drug Delivery, 27(1), 681-690. DOI