ON THE QUESTION OF THE MOLECULAR AND GENETIC MECHANISMS BY WHICH BONE TISSUE OSTEOBLASTS INTERACT WITH BIOLOGICAL MATERIALS DURING OSTEOPLASTY
Samara State Medical University, Biotech Research Institute, 89 Chapaevskaya str,. Samara, 443079 Russia; *e-mail: tatjana.medv@rambler.ru
Keywords: osteoblasts; cell adhesion; activation of reparative processes; signaling pathways and enzymatic reaction cascades
DOI:10.18097/BMCRM00262
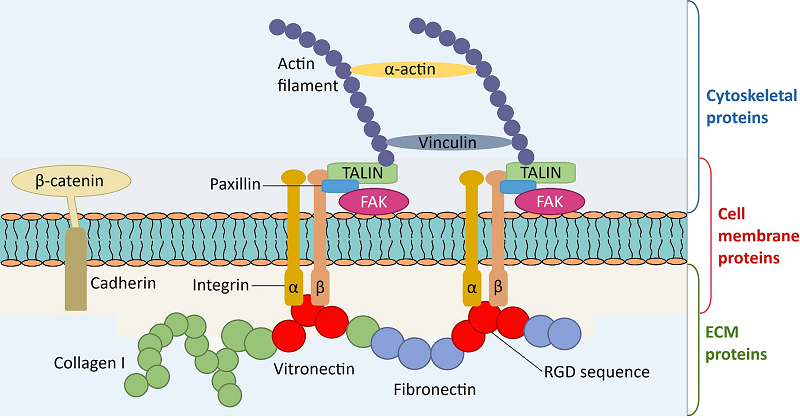
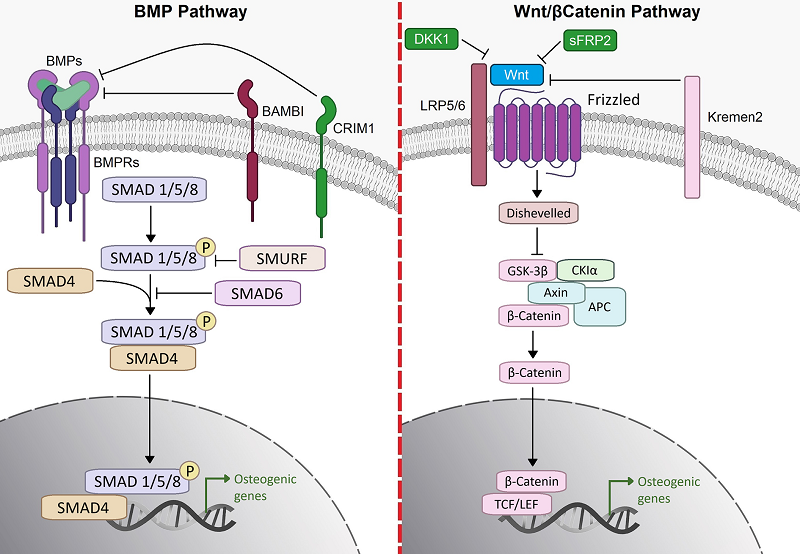
The article herein sets out to show the molecular and genetic mechanisms underpinning regenerative processes in bone tissue during the implantation of biological materials. The adhesion of osteoblasts to biological materials is a pivotal step in the transfer of physicochemical signals from biomaterials to osteoblasts. Initially, bone tissue cells interact with the biological material indirectly, through specific extracellular matrix proteins, especially vitronectin, fibronectin, and type I collagen. During the period preceding direct contact of osteoblasts with the implant, it is possible for blood proteins to be absorbed on the surface of the biological material in few seconds. Exactly the formation of a "protein layer" on the implants has been demonstrated to favour the adhesion of osteoblasts. The process of adhesion of osteoblasts to blood proteins is facilitated by a specific sequence, RGD, a tripeptide consisting of the amino acids Arg, Gly and Asp. This sequence is characteristic of vitronectin, fibronectin, type I collagen, osteopontin, bone sialoprotein and thrombospondin. Integrin-related signalling pathways can be categorised into two distinct types: those that are contingent on the Src-FAK complex and those that are not. It has been established that the phosphorylation of FAK at various sites can trigger multiple signalling pathways, including the PI3K/Akt/mTOR pathway, the Ras/MAPK/ERK1/2 pathway, and the p130Cas-RhoA GTPase pathway. For signalling pathways whose action is not contingent on the Src-FAK complex, integrin-linked kinase (ILK) is a pivotal regulatory factor. Among other significant cell membrane proteins, cadherins have been demonstrated to function as signal transduction molecules, contributing to the regulation of critical cellular activities. Consequently, in addition to the physical attachment of osteoblasts to biomaterials, cell adhesion leads to the activation of several signalling pathways, among which integrin- and cadherin-related signalling pathways are the most significant.

|
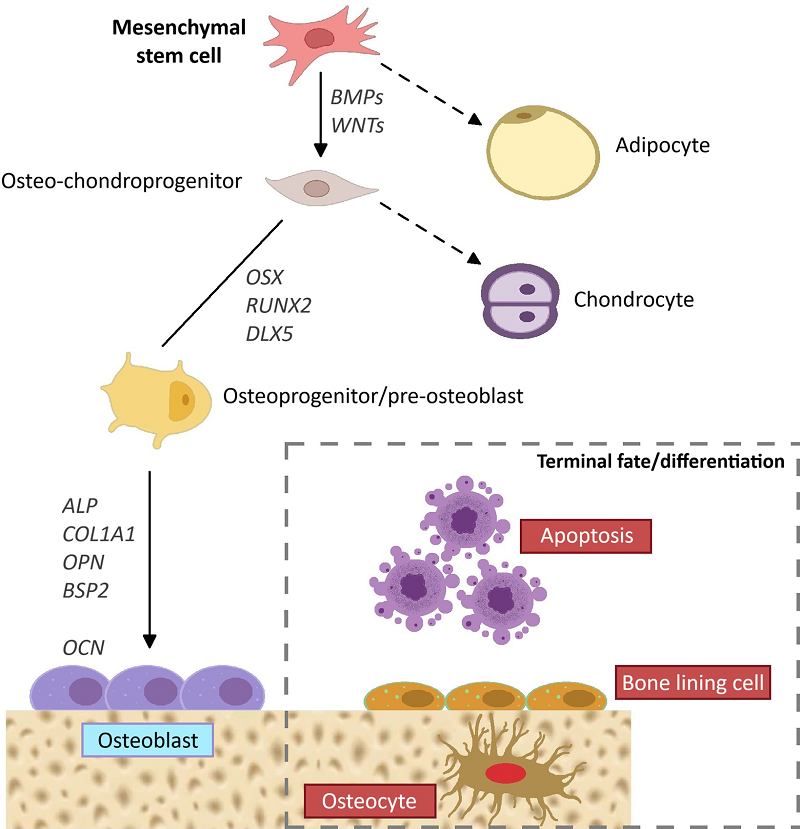
Figure 1.
MSC differentiation. The possible differentiation ways of mesenchymal stem cells. Development of osteoblasts and the appearance of osteocytes ([23]).
|
FUNDING
No external funding.
REFERENCES
- Ogle, O.E. (2015) Implant surface material, design, and osseointegration.Dent. Clin. North Am., 59(2), 505-520. DOI
- Chen, Ni, S., Han, S., Crauford, R., Lu, S., Wei, F., Chang, J., Wu, C., Xiao,Y. (2017) Nanoporous microstructures mediate osteogenesis by modulating theosteo-immune response of macrophages. Nanoscale, 9(2), 706-718. DOI
- Chen, Z., Bachhuka, A., Wei, F., Wang, X., Liu, G., Vasilev, K., Xiao, Y.(2017) Nanotopography-based strategy for the precise manipulation ofosteoimmunomodulation in bone regeneration. Nanoscale, 9(46), 18129-18152. DOI
- Chen, Sh., Guo, Y., Liu, R., Wu, S., Fang, J., Huang, B., Li, Z., Chen, Zh.,Chen, Z. (2018) Tuning surface properties of bone biomaterials to manipulateosteoblastic cell adhesion and the signaling pathways for the enhancement ofearly osseointegration. Coll. and Surf. B: Biointerfaces, 164, 58-69. DOI
- Vagaska, B., Bacakova, L., Filova, E., Balik, K. (2010) Osteogenic cells onbio-inspired materials for bone tissue engineering. Physiol. Res., 59(3), 309-322. DOI
- Rico, P., Rodriguez Hernandez, J.C., Moratal, D., Altankov, G., Monleon,P.M., Salmeronsanchez, M. (2009) Substrate-induced assembly of fibronectininto networks: influence of surface chemistry and effect on osteoblast adhesion.Tissue Eng. Part A, 15(11), 3271-3281. DOI
- Hidalgo-Bastida, L.A., Cartmell, S.H. (2010) Mesenchymal stem cells,osteoblasts and extracellular matrix proteins: enhancing cell adhesion anddifferentiation for bone tissue engineering. Tissue Eng. Part B Rev., 16(4), 405-412. DOI
- Zhou, J., Han, Y., Lu, S. (2014) Direct role of interred spacing in mediatingcell adhesion on Sr-HA nanorod-patterned coatings. Int. J. Nanomedicine, 9,1243-1260. DOI
- Khalilii, A.A., Ahmad, M.R. (2015) A review of cell adhesion studies forbiomedical and biological applications. Int. J. Mol. Sci., 16(8), 18149-18184. DOI
- Kim, S.H., Turnbull, J., Guimond, S. (2011) Extracellular matrix and cellsignaling: the dynamic cooperation of integrin, proteoglycan and growth factorreceptor. J. Endocrinol., 209(2), 139-151. DOI
- Manning, B.D., Cantley, L.C. (2007) AKT/PKB signaling: navigatingdownstream. Cell, 129(7), 1261-1274. DOI
- Guntur, A.R., Rosen, C.J. (2011) New insights into osteoblasts and their rolein bone formation: the central role of P13 kinase. J. Endocrinol., 211(2), 123-130. DOI
- Mitra, S.K., Hanson, D.A., Schlaepfer, D.D. (2005) Focal adhesion kinase:in command and control of cell motility. Nat. Rev. Mol. Cell Biol., 6(1), 56-68. DOI
- Jiang, T., Guo, L., Ni, S., Zhao, Y. (2015) Upregulation of cell proliferationvia Shс and ERK1/2 MAPK signaling in SaOS-2 osteoblasts grown onmagnesium alloy surface coating with tricalcium phosphate. J. Mater. Sci.Mater. Med., 26(4), 158. DOI
- Ge, C., Yang, Q., Zhao, G., Yu, H., Kirkwood, K.L., Franceschi, R.T. (2012)Interactions between extracellular signal-regulated kinase 1/2 and p38 MAPkinase pathways in the control of RUNX2 phosphorylation and transcriptionalactivity. J. Bone Miner. Res., 27(3), 538-551. DOI
- Schroeder, T.M., Jensen, E.D., Westendorf, J.J. (2005) Runx2: a masterorganizer of gene transcription in developing and maturing osteoblasts. Birth.Defects Research (Part C), 75(3), 213-225. Wiley-Liss, Inc. DOI
- Legate, K.R., Wickstrӧm, S.A., Fӓssler, R. (2009) Genetic and cell biologicalanalysis of integrin outside-in signaling. Genes Dev., 23(4), 397-418. DOI
- Dejaeger, M., Bohm, A.M., Dirckx, N., Devriese, J., Nefyodova, E., Cardoen,R., St-Arnaud, R., Tournoy, J., Luyten, F.P., Maes, C. (2017) Integrin-linkedkinase regulates bone formation by controlling cytoskeletal organization andmodulating BMP and Wnt signaling in osteoprogenitors. J. Bone Miner. Res., 32(10), 2087-2102. DOI
- Xu, L., Meng, F., Ni, M., Lee, Y., Li, G. (2013) N-cadherin regulatesosteogenesis and migration of bone marrow-derived mesenchymal stem cells.Mol. Biol. Rept., 40(3), 2533-2539. DOI
- Marie, P.J., Hay, E., Modrowski, D., Revollo, L., Mbalaviele, G., Civitelli,R. (2014) Cadherin-mediated cell-cell adhesion and signaling in the skeleton.Calcif. Tissue Int., 94(1), 46-54. DOI
- Vlashi, R., Zhang, X., Wu, M., Chen, G. (2023) Wnt signaling: essentialroles in osteoblast differentiation, bone metabolism and therapeutic implicationsfor bone and skeletal disorders. Gene Dis., 10(4), 1291-1317. DOI
- Liu, J., Xiao, Q., Xiao, J., Niu, Ch., Li, Yu., Zhang, X., Zhou, Zh., Shu, G.,Yin, G. (2022) Wnt/β-catenin signaling function, biological mechanisms, andtherapeutic opportunities. Signal transduction and targeted therapy, 7(1), 3. DOI
- Ponzetti, M., Rucci, N. (2021) Osteoblast differentiation and signaling:established concepts and emerging topics. Int. J. Mol. Sci., 22(13), 6651. DOI
- Cech, T.R., Steitz, J.A. (2014) The noncoding RNA revolution—trashing oldrules to forge new ones. Cell, 157, 177–194. DOI
- Capulli, M., Paone, R., Rucci, N. (2014) Osteoblast and osteocyte: gameswithout frontiers. Arch. Biochem. Biophys., 561, 563–612. DOI
- Boyden, L.M., Mao, J., Belsky, J., Mitzner, L., Farhi, A., Mitnick, M.A., Wu,D., Insogna, K., Lifton, R.P. (2002) High bone density due to a mutation inLDL-receptor–related protein 5. N. Engl. J. Med., 346, 1513–1521. DOI
- Zhang, J., Hao, X., Yin, M., Xu, T., Guo, F. (2019) Long Non-Coding RNAin osteogenesis: a new world to be explored Bone Jt. Res., 8, 73–80. DOI
- Nardocci, G., Carrasco, M.E., Acevedo, E., Hodar, C., Meneses, C.,Montecino, M. (2018) Identification of a novel long noncoding RNA thatpromotes osteoblast differentiation. J. Cell Biochem., 119, 7657–7666. DOI
- Zhang, W., Dong, R., Diao, S., Du, J., Fan, Z., Wang, F. (2017) Differentiallong noncoding RNA/mrna expression profiling and functional network analysisduring osteogenic differentiation of human bone marrow mesenchymal stemcells. Stem Cell Res. Ther., 8, 1–13. DOI
- Wei, B., Wei, W., Zhao, B., Guo, X., Liu, S. (2017) Long non-codingRNA HOTAIR inhibits mir-17-5p to regulate osteogenic differentiation andproliferation in nontraumatic osteonecrosis of femoral head. PLoS One, 12(2),e0169097. DOI
- Jia, J., Feng, X., Xu, W., Yang, S., Zhang, Q., Liu, X., Dai, Y.F.Z. (2014)MiR-17-5p modulates osteoblastic differentiation and cell proliferation bytargeting SMAD7 in non-traumatic osteonecrosis. Exp. Mol. Med., 46. DOI
- .Silva, A.M., Moura, S.R., Teixeira, J.H., Barbosa, M.A., Santos, S.G.,Almeida, M.I. (2019) Long noncoding RNAs: a missing link in osteoporosis.Bone Res., 7, 1–16. DOI
- Barrett, S.P., Salzman, J. (2016) Circular RNAs: analysis, expression andpotential functions. Development, 143, 1838–1847. DOI
- Ouyang, Z., Tan, T., Zhang, X., Wan, J., Zhou, Y., Jiang, G., Yang, D., Guo,X., Liu, T. (2019) CircRNA hsa_circ_0074834 promotes the osteogenesisangiogenesiscoupling process in bone mesenchymal stem cells (BMSCs) byacting as a ceRNA for miR-942-5p. Cell Death Dis., 10. DOI
- Huang, X., Cen, X., Zhang, B., Liao, Y., Zhu, G., Liu, J., Zhao, Z. (2019)Prospect of circular RNA in osteogenesis: a novel orchestrator of signalingpathways. J. Cell. Physiol., 234, 21450–21459. DOI
- Tsiklin, I.L., Pugachev, E.I., Kolsanov, A.V., Timchenko, E.V., Boltovskaya,V.V., Timchenko, P.E., Volova, L.T. (2022) Biopolymer material from humanspongiose for regenerative medicine application. Polymers, 14(5), 941. DOI
- Medvedeva, T., Volova, L., Kulagina, L. (2023) The study of type I collagenby immunoblotting in samples of bone-plastic biomaterials. BiomedicalChemistry: Research and Methods, 6(2), e00189. DOI
- Ryabov, N.A., Volova L.T., Alekseev D.G., Kovaleva S.A., Medvedeva T.N.,Vlasov M.Yu. (2024) Mass spectrometry of collagen – containing allogeneichuman bone tissue material. Polymers (Basel), 16(13), 1895. DOI