ASSOCIATION OF NADPH-OXIDASE 2 (NOX2) WITH THE DEVELOPMENT OF ATOPIC ASTHMA
1Kazan (Volga Region) Federal University, Kremlevskaya st. 18; Kazan, 420008 Russia; *e-mail: Ibragimov94@inbox.ru
2Kazan Research Institute of Epidemiology and Microbiology, Kazan
Keywords: NADPH–oxidase 2 (NOX2); LC3–associated phagocytosis моноциты; atopic bronchial asthma
DOI:10.18097/BMCRM00272
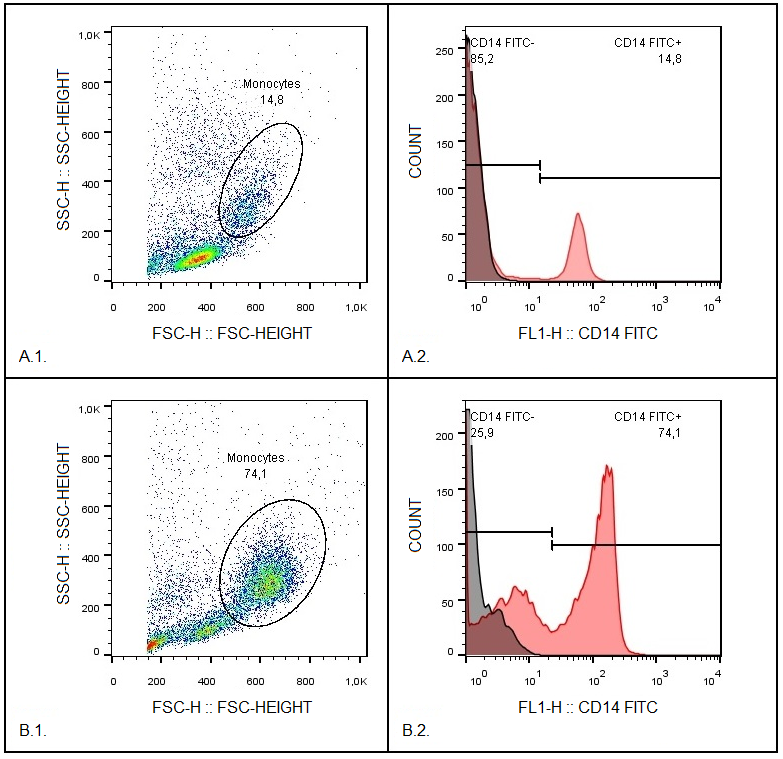
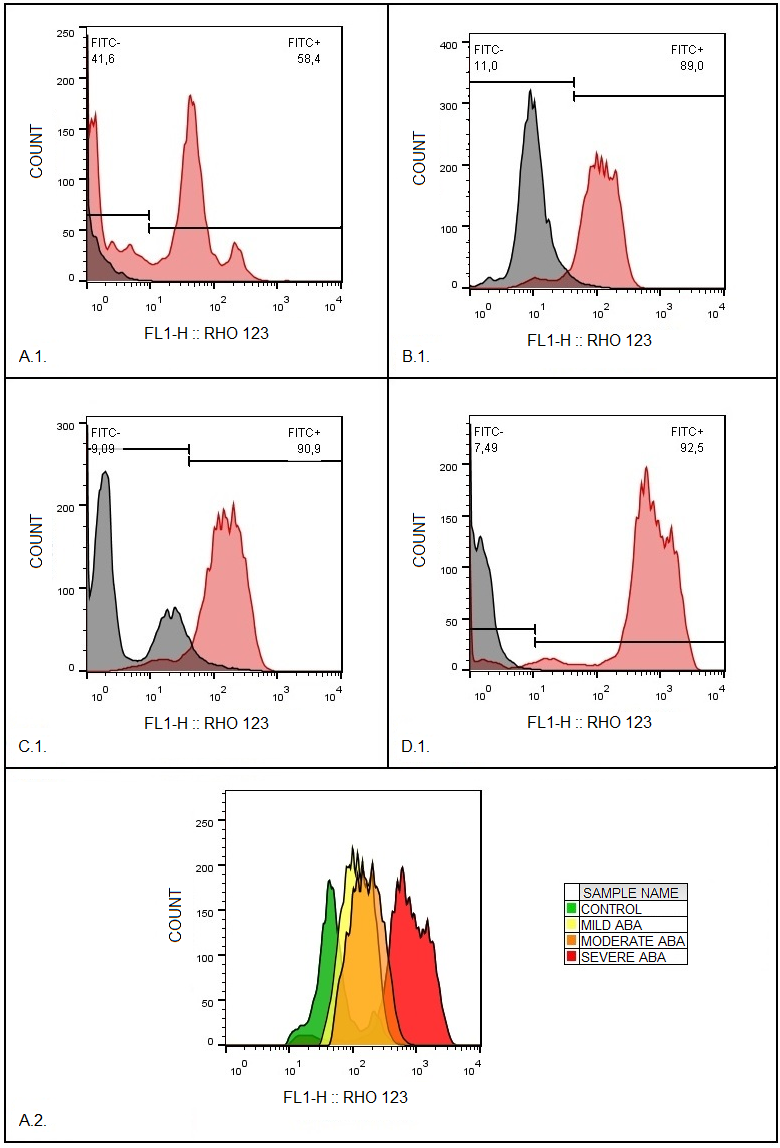
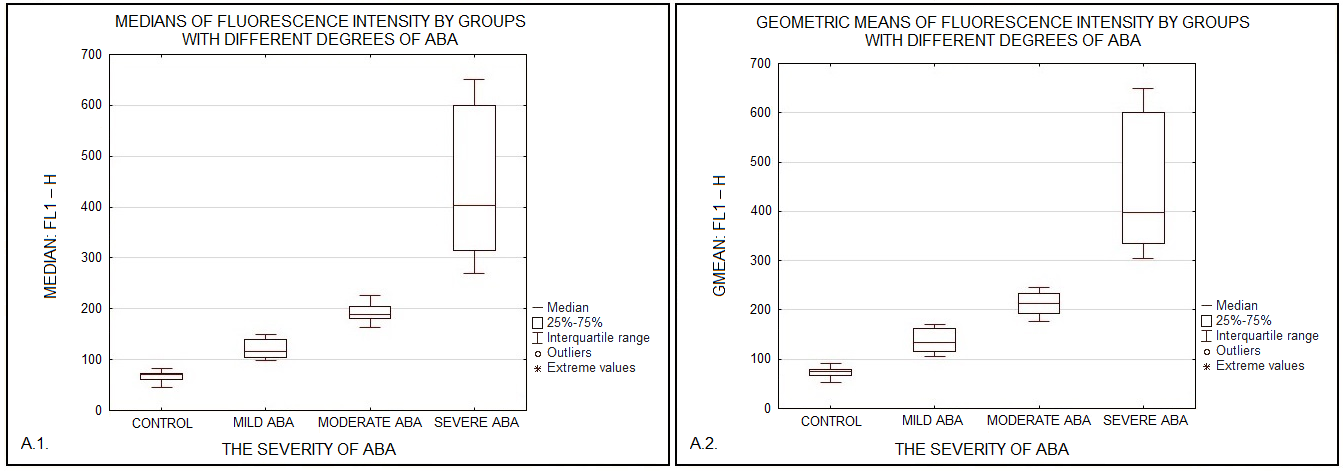
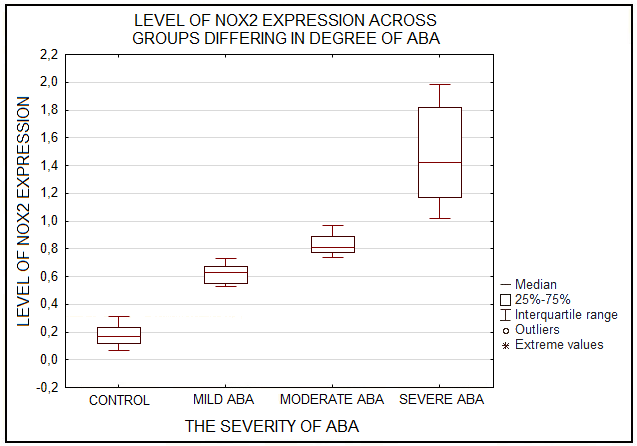
Phagocytic cells destroy bacteria, allergens, and apoptotic cells via antimicrobial reactions coupled with phagocytosis, including generation of reactive oxygen species (ROS) and delivery of hydrolytic enzymes from lysosomes to the phagosome. ROS produced by NADPH oxidase 2 (NOX2), are involved in the mechanism of LC3-associated phagocytosis; they participate in the development of innate and adaptive immune responses, leading to airway remodeling and hyperreactivity. The aim of this study was to investigate an association between NOX2 and the severity of atopic bronchial asthma (ABA). The level of ROS was determined in monocytes of healthy donors and patients with atopic bronchial asthma using dihydrorhodamine 123 (DHR-123). The expression level of NOX2 gene in monocytes of healthy donors and patients with atopic bronchial asthma was analyzed by RT-PCR. In the presence of PMA (phorbol 12-myristate 13-acetate), there was a significant increase in rhodamine-123 (Rho-123) fluorescence in all studied groups. At the same time, a significant increase in the median of Rho-123 fluorescence was found in the group with severe ABA as compared to healthy donors and patients with mild AAA, as well as to the group of patients with moderate ABA. A significant increase in NOX2 gene expression was found in the groups with moderate and severe ABA compared to the other studied groups. Based on the data obtained, it can be assumed that with an increase in the activity of NOX2, the key enzyme of LC3-associated phagocytosis, there is an aggravation of the course of ABA.

|
Figure 4.
Diagram of NOX2 expression levels in monocytes of healthy donors (control) and patients with varying degrees of atopic bronchial asthma (ABA) determined by RT-PCR.
|
Table 1. Primers used for RT-PCR to determine the expression level of NOX2 gene in monocytes from healthy donors and patients with atopic bronchial asthma.
|
Primers |
Primer sequence (5’ → 3’) |
Primer size (base pairs) |
|
NOX2 Forward primer |
AAGCCTTGGCTGAAACCCTG |
20 |
|
NOX2 Reverse primer |
TTGAAAATGAAATGCACTCCCCG |
23 |
|
B2M Forward primer |
CCACTGAAAAAGATGAGTATGCCT |
24 |
|
B2M Reverse primer |
CCAATCCAAATGCGGCATCTTCA |
23 |
FUNDING
The work was carried out within the framework of the program "Strategic Academic Leadership of Kazan Federal University" (PRIORITY-2030).
REFERENCES
- Mak, J.C., Leung, H.C., Ho, S.P., Law, K.W.B., Lam, W.K., Tsang, K.W.T., Ip,M.S.M., Chan–Yeung, M. (2004) Systemic oxidative and antioxidative status inChinese patients with asthma. J. Allergy Clin. Immunol., 114(2), 260–264. DOI
- Sweet, M.J., Ramnath, D., Singhal, A., Kapetanovic, R. (2024) Inducibleantibacterial responses in macrophages. Nature Reviews Immunology, 25(2),92-107. DOI
- Ibragimov, B.R., Skibo, Yu.V., Abramova, Z.I. (2023) Autophagy and LC3–associated phagocytosis: similarities and differences. Medical Immunology(Russia), 25(2), 233–252. DOI
- Kim, B.H., Shenoy, A.R., Kumar, P. (2011) A family of IFN-gamma-inducible65-kD GTPases protects against bacterial infection. Science, 332(6030),717–721. DOI
- Matsunaga, K., Saitoh, T., Tabata, K., Omori, H., Satoh, T., Kurotori, N.,Maejima, I., Shirahama-Noda, K., Ichimura, T., Isobe T., Akira, S., Noda, T.,Yoshimori, T. (2009) Two Beclin 1-binding proteins, Atg14L and Rubicon,reciprocally regulate autophagy at different stages. Nat. Cell Biol., 11(4),385–396. DOI
- Qu, J., Li, Y., Zhong, W., Gao, P, Hu, C.J. (2002) Recent developments in therole of reactive oxygen species in allergic, asthma. J. Thorac. Dis., 9(1), E32–E43. DOI
- Michaeloudes, C., Abubakar–Waziri, H., Lakhdar, R., Raby, K., Dixey,P., Adcock, I.M., Mumby, S., Bhavsar, P.K., Chung, K.F. (2022) Molecularmechanisms of oxidative stress in asthma. Molecular Aspects of Medicine, 85,e101026. DOI
- Ravikumar, B., Moreau, K., Jahreiss, L., Puri, C., Rubinsztein, D.C. (2010)Plasma membrane contributes to the formation of pre-autophagosomalstructures. Nature Cell Biology, 12(8), 747-757. DOI
- Ammar, M., Bahloul, N., Amri, O., Omri, R., Ghozzi, H., Kammoun, S.,Zeghal, K., Mahmoud, L.B. (2022) Oxidative stress in patients with asthma andits relation to uncontrolled asthma. J. Clin. Lab. Anal., 36(5), e24345. DOI
- Karadogan, B., Beyaz, S., Gelincik, A., Buyukozturk, S., Arda, N. (2022)Evaluation of oxidative stress biomarkers and antioxidant parameters inallergic asthma patients with different level of asthma control. J. Asthma, 59(4),663–672. DOI
- Nadeem, A., Chhabra, S.K., Masood, A., Raj, H.G. (2003) Increasedoxidative stress and altered levels of antioxidants in asthma. J. Allergy Clin.Immunol., 111(1), 72–78. DOI
- Asrar, A., Shameem, M., Husain, Q. (2012) Relation of oxidant antioxidantimbalance with disease progression in patients with asthma. Ann. Thorac Med.,7(4), 226–232. DOI
- Anes, A.B., Nasr, H.B., Fetoui, H., Bchir, S., Chahdoura, H., Yacoub, S.,Garrouch, A., Benzarti, M., Tabka, Z., Chahed, K. (2015) Alteration in systemicmarkers of oxidative and antioxidative status in Tunisian patients with asthma:relationships with clinical severity and airflow limitation. J. of Asthma, 53(3),227–237. DOI
- Asare, P.F., Hurtado, P.R., Tran, H.B., Perkins, G.B., Roscioli, E., Hodge,S. (2023) Reduction in Rubicon by cigarette smoke is associated with impairedphagocytosis and occurs through lysosomal degradation pathway. Clinical andExperimental Medicine, 23(7), 4041–4055. DOI
- Levy, M.L., Bacharier, L.B., Bateman, E., Boulet, L.P., Brightling, C., Buhl,R., Brusselle, G., Cruz, A.A., Drazen, J.M., Duijts, L., Fleming, L., Inoue, H.,Ko, F.W.S., Krishnan, J.A., Mortimer, K., Pitrez, P.M., Sheikh, A., Yorgancıoğlu,A., Reddel, H.K. (2023) Key recommendations for primary care from the 2022Global Initiative for Asthma (GINA) update. N.P.J. Prim. Care Respir. Med.,33(1), 7. DOI
- Gassan, D.A., Kotova, O.O., Naumov, D.E., Sugaylo, I.Yu., Gorchakova,Y.G., Sinyuk, A.A. (2020) Comparative characteristics of monocytes isolationconditions by adhesion method for in vitro experiments. Bulletin Physiologyand Pathology of Respiration, 78, 128-134. DOI
- Treves, A.J., Yagoda, D., Haimovitz, A., Ramu, N., Rachmilewitz, D., Fuks,Z. (1980) The isolation and purification of human peripheral blood monocytesin cell suspension. J. Immunol., 39(2), 71–80. DOI
- Yang, Y., Zhang, G., Yang, T., Gan, J., Xu, L., Yang, H. (2018) A flow–cytometry–based protocol for detection of mitochondrial ROS production underhypoxia. Star Protocols, 2(2), e100466. DOI
- Assing, K., Laursen, C.B., Campbell, A.J., Beck, H.C., Davidsen, J.R. (2024)Proteome and dihydrorhodamine profiling of bronchoalveolar lavage in patientswith chronic pulmonary aspergillosis. J. Fungi, 10(5), 314. DOI
- Richardson, M.P., Ayliffe, M.J., Helbert, M., Davies, E.G. (1998) A simpleflow cytometry assay using dihydrorhodamine for the measurement of theneutrophil respiratory burst in whole blood: comparison with the quantitativenitrobluetetrazolium test. Journal of Immunological Methods, 219(1), 187–193. DOI
- Pioch, J., Blomgran, R. (2022) Optimized flow cytometry protocol fordihydrorhodamine 123–based detection of reactive oxygen species in leukocytesubpopulations in whole blood. Journal of Immunological Methods, 507,e113308. DOI
- Vernon, P.J., Schaub, L.J., Dallelucca, J.J., Pusateri, A.E., Sheppard, F.R.(2015) Rapid detection of neutrophil oxidative burst capacity is predictive ofwhole blood cytokine responses. PLoS One, 10(12), 663–672. DOI
- Johnstone, A.M., Koh, A., Goldberg, M.B., Glogauer, M. (2007) Ahyperactive neutrophil phenotype in patients with refractory periodontitis. J.Periodontol., 78(9), 1788–1794. DOI
- Siddiqi, M., Garcia, Z.C., Stein, D.S., Denny, T.N., Spolarics, Z. (2001)Relationship between oxidative burst activity and CD11b expression inneutrophils and monocytes from healthy individuals: Effects of race and gender.Cytometry, 46(4), 243–246. DOI
- Bylund, J.J., Brown, K.L., Movitz, C., Dahlgren, C., Karlsson, A. (2010)Intracellular generation of superoxide by the phagocyte NADPH oxidase: how,where, and what for? Free Radic. Biol. Med., 49(12), 1834–1845. DOI
- Kavianpour, M., Moradzadeh, K., Muhammadnejad, S., Jabbarpour, Z.,Khorsand, A.A., Aghayan, S., Vasei, M., Verdi, J. (2022) Flow cytometricmeasurement of reactive oxygen species to assess the effects of preconditioningtotal body irradiation on NOG mice. Asian Pac. J. Cancer Prev., 23(2), 383–382. DOI
- Quach, A., Glowik, S., Putty, T., Ferrante, A. (2019) Delayed bloodprocessing leads to rapid deterioration in the measurement of the neutrophilrespiratory burst by the dihydrorhodamine–123 reduction assay. Cytometry BClin. Cytom., 96(5), 389–396. DOI
- Chang, S.C., Rodrigues, N.P., Zurgil, N., Henderson, J.R., Bedioui, F.,McNeil, C.J., Deutsch, M. (2005) Simultaneous intra– and extracellularsuperoxide monitoring using an integrated optical and electrochemical sensorsystem. Biochem. Biophys. Res. Commun., 327(4), 979–984. DOI
- Ghasemzadeh, M., Hosseini, E. (2017) Platelet granule release is associatedwith reactive oxygen species generation during platelet storage: A direct linkbetween platelet pro–inflammatory and oxidation states. Thromb. Res., 156(5),101–104. DOI
- Vernon, P.J., Paredes, R.M., Sooter, A.J., Schaub, L.J., Grossman, H.M.,Pusateri, A.E., Glaser, J.J., Sheppard, F.R. (2016) Severe hemorrhagic shockinduces acute activation and expansion of IL–8+/IL–10+ neutrophils withenhanced oxidative reactivity in non–human primates. Shock, 46(3), 129–136. DOI
- Hastuti, S.D., Quach, A., Costabile, M., Barton, M.D., Pyecroft, S.B.,Ferrante A. (2019) Measuring the Asian seabass (Lates calcarifer) neutrophilrespiratory burst activity by the dihydrorhodamine–123 reduction flowcytometry assay in whole blood. Fish Shellfish Immunol., 92(5), 871–880. DOI
- Djiadeu, P., Azzouz, D., Khan, M.A., Kotra, L.P., Sweezey, N., Palaniyar, N.(2017) Ultraviolet irradiation increases green fluorescence of dihydrorhodamine(DHR–) 123: false–positive results for reactive oxygen species generation.Pharmacol. Res. Perspect., 5(2), e00303. DOI
- Wardman, P. (2008) Methods to measure the reactivity of peroxynitrite–derived oxidants toward reduced fluoresceins and rhodamines. MethodsEnzymol., 441, 261–282. DOI
- Vowells, S.J., Sekhsaria, S., Malech, H.L., Shalit, M., Fleisher, T.A. (1995)Flow cytometric analysis of the granulocyte respiratory burst: a comparisonstudy of fluorescent probes. J. Immunol. Methods, 178(1), 89–97. DOI
- Tichopad, A., Dilger, M., Schwarz, G., Pfaffl, M.W. (2003) Standardizeddetermination of real–time PCR efficiency from a single reaction set–up.Nucleic Acids Res., 31(20), e122. DOI
- Bantula, M, Arismendi, E., Picado, C., Mullol, J., Roca-Ferrer, J., Tubita,V. (2022) Reference Gene Validation for RT–qPCR in PBMCs from AsthmaticPatients with or without Obesity. Methods Protoc., 5(3), E35. DOI
- Ibragimov, B.R., Skibo, Yu.V., Reshetnikova, I.D., Abramov, S.N., Daminova,A.G., Evtyugin, V.G., Abramova Z.I. (2024) Effect of the Rubicon protein onLC3-associated phagocytosis by monocytes in the patients with severe atopicbronchial asthma. Medical Immunology (Russia), 26(6), 1213–1222. DOI
- Wong, S., Payel Sil, P., Martinez, J. (2017) Rubicon: LC3-associatedphagocytosis and beyond. The FEBS journal, 285(8), 1379-1388. DOI