SUPEROXIDIZING AND ANTIOXIDANT ACTIVITY OF NICOTINAMIDE COENZYMES IN VITRO
Institute of Theoretical and Experimental Biophysics, Russian Academy of Sciences, Institutskaya ul. 3, Pushchino, Moscow Region, 142290, Russia; *e-mail: sirotatv@rambler.ru
Keywords: nicotinamide coenzyme; NAD; NADP; NADH; NADPH; superoxide, nitroblue tetrazolium
DOI:10.18097/BMCRM00276
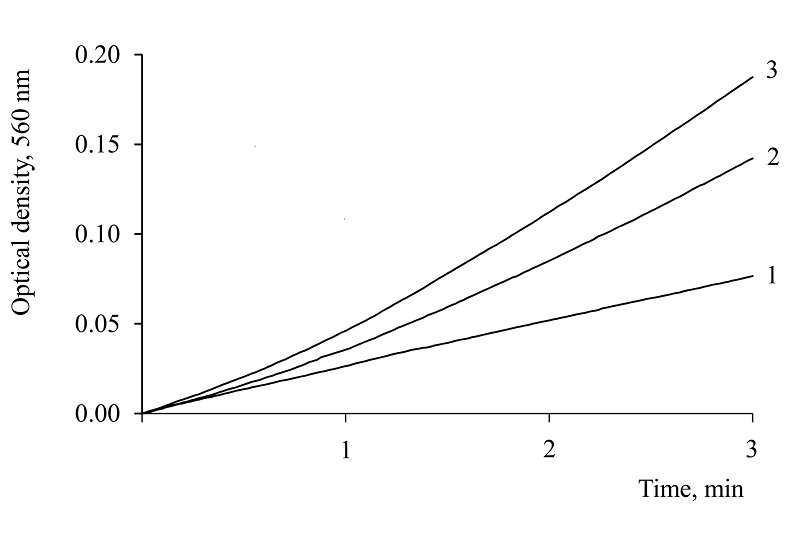
New properties of nicotinamide coenzymes (NAD, NADP, NADH, NADPH) have been discovered: superoxide-generating and antioxidant activities. Coenzymes are capable of generating superoxide anions (O2─●), entering an alkaline environment; and can be O2─● traps, inhibiting the generation process in superoxide-generating model systems, thus exhibiting antioxidant properties. Actually, nicotinamide itself, which is a functional part in the coenzyme molecule in oxidation-reduction processes, showed only antioxidant activity in vitro. Antioxidant properties have also been found in adenosine, which is part of the coenzyme molecule. Thus, it has been established for the first time that coenzymes, according to their chemical properties, are bifunctional molecules. It is assumed that they participate in cellular signaling through the generation of superoxide and, in the case of excess superoxide in the environment, they can be antioxidants. All these properties of coenzymes and their components must be taken into account when used in scientific research and medicine.

|
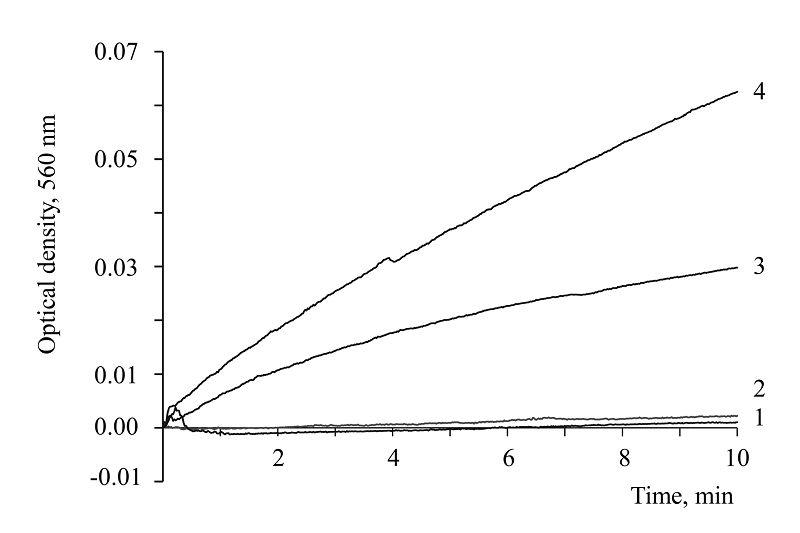
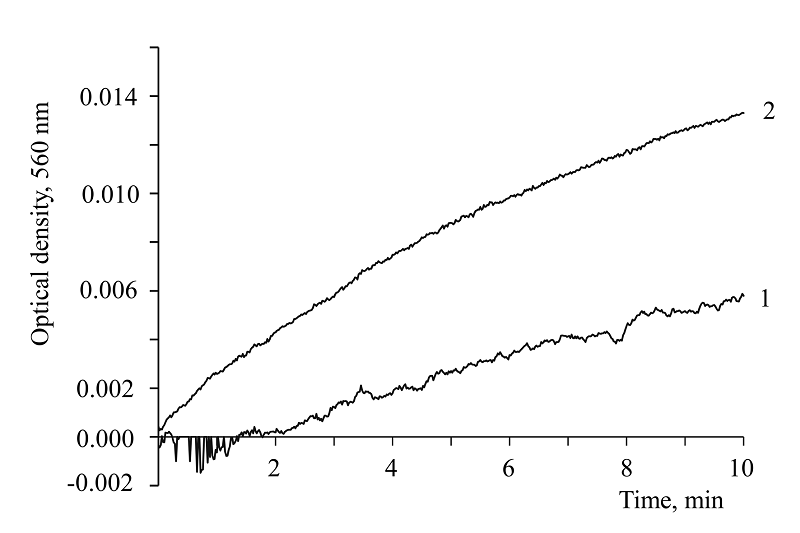
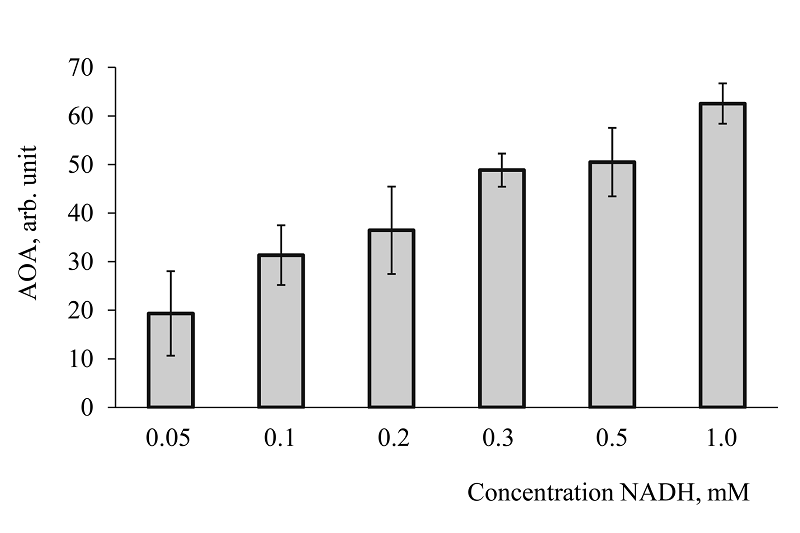
Figure 6.
Antioxidant activity of different NADH concentrations.
Measurement conditions: 0.2 M carbonate buffer, pH 10.6; 0.05 mM NBT, 0.058 mM adrenaline. Temperature 20°C.
|
|
CLOSE

|
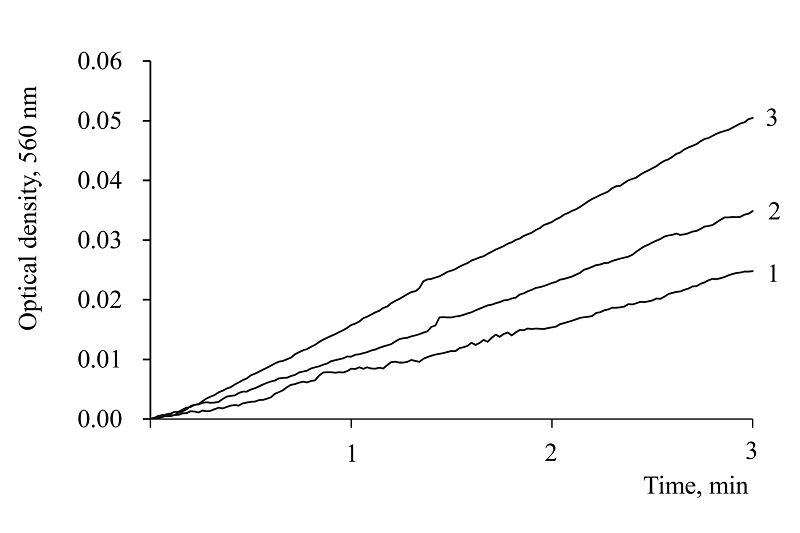
Table 1.
Antioxidant activity of the studied compounds in the enzymatic reaction xanthine-xanthine oxidase.
|
FUNDING
The work was carried out within the framework of the State Assignment of ITEB RAS No. 075-00223-25-00.
REFERENCES
- Koju, N., Qin, Z., Sheng, R. (2022) Reduced nicotinamide adeninedinucleotide phosphate in redox balance and diseases: a friend or foe? ActaPharmacologic Sinica, 43, 1889–1904. DOI
- Kirsch, M., Groot, H. (2001) NAD(P)H, a directly operating antioxidant?FASEB J., 15(9), 1569-1574. DOI
- Ying, W. (2006) NAD+ and NADH in cellular functions and cell death. Front.Biosci., 11, 3129– 3148. DOI
- Ray, P.D., Huang, B.W., Tsuji, Y. (2012) Reactiv oxygen species (ROS)homeostasis and redox regulation in cellular signaling. Cell Signal., 24(5),981–990. DOI
- Schieber, M., Chandel, N.S. (2014) ROS function in redox signaling andoxidative stress. Curr. Biol., 24(10), R453-462. DOI
- Andrés, C.M.C, Pérez de la Lastra, J.M., Andrés Juan, C., Plou, F.J., Pérez-Lebeña, E. (2023) Superoxide anion chemistry - its role at the core of the innateimmunity. Int. J. Mol. Sci., 24(3), 1841. DOI
- Sirota, T. (2023) Superoxide generation by nicotinamide coenzymes.Biomedical Chemistry: Research and Methods, 6(1), e00188. DOI
- Sirota, T.V. (2024) Superoxide generating activity of nicotinamidecoenzymes. Biophysics, 69, 18–24. DOI
- Guilbert, C.C., Johnson, S.L. (1971) Isolation and characterization of thefluorescent alkali product from diphosphopyridine nucleotide. Biochemistry,10(12), 2313-2316. DOI
- Metzler, D.E., Metzler, C.M. (2003) Biochemistry: the chemical reactions ofliving cells, 2nd Edition, Academic Press, New York, 1, 1973 p.
- Altman, F.P. (1976) Tetrazolium salts and formazans. Progress inHistochemistry and Cytochemistry, 9(3), 1-56. DOI
- Beauchamp, C., Fridovich, I. (1971) Superoxide dismutase: improvedassays and an assay applicable to acrylamide gels. Anal Biochem, 44(1), 276-87. DOI
- Sirota, T.V. (2012) Use of nitro blue tetrazolium in the reaction ofadrenaline autooxidation for the determination of superoxide dismutase activity.Biomeditsinskaya Khimiya, 58(1), 77-87. DOI
- Sirota, T.V. (2016) Standardization and regulation of the rate of thesuperoxide-generating adrenaline autoxidation reaction used for evaluation ofpro/antioxidant properties of various materials. Biomeditsinskaya Khimiya,62(6), 650-655. DOI
- Sirota, T.V., Sirota, N.P. (2022) On the Mechanism of Oxygen Activation inChemical and Biological Systems. Biophysics, 67(1), 1–7. DOI
- Sirota, T.V. (2020) A chain reaction of adrenaline autoxidation is a model ofquinoid oxidation of catecholamines. Biophysics, 65, 548–556. DOI
- Hayyan, M., Hashim, M.A., AlNashef, I.M. (2016) Superoxide ion:generation and chemical implications. Chem Rev., 116(5), 3029-85. DOI
- Ma, Y., Nie, H., Chen, H., Li, J., Hong, Y., Wang, B., Wang, C., Zhang, J.,Cao, W., Zhang, M., Xu, Y., Ding, X., Yin, S.K., Qu, X., Ying, W. (2015) NAD+/NADH metabolism and NAD+-dependent enzymes in cell death and ischemicbrain injury: current advances and therapeutic implications. Curr. Med. Chem.,22(10), 1239-1247. DOI
- Kamal, S., Babar, S., Ali, W., Rehman, K., Hussain, A., Akash, M.S.H. (2024)Sirtuin insights: bridging the gap between cellular processes and therapeuticapplications. Naunyn-Schmiedeberg’s Arch. Pharmacol., 397, 9315–9344. DOI
- Wang, J., Zhao, C., Kong, P., Sun, H., Sun, Z., Bian, G., Sun, Y., Guo,L. (2016) Treatment with NAD(+) inhibited experimental autoimmuneencephalomyelitis by activating AMPK/SIRT1 signaling pathway andmodulating Th1/Th17 immune responses in mice. Int. Immunopharmacol., 39,287-294. DOI
- Lu, L., Tang, L., Wei, W., Hong, Y., Chen, H., Ying, W., Chen, S. (2014)Nicotinamide mononucleotide improves energy activity and survival rate in anin vitro model of Parkinson’s disease. Exp. Ther. Med., 8(3), 943-950. DOI
- Wang, J., Song, X., Tan, G., Sun, P., Guo, L., Zhang, N., Wang, J., Li, B.(2021) NAD+ improved experimental autoimmune encephalomyelitis byregulating SIRT1 to inhibit PI3K/Akt/mTOR signaling pathway. Aging (AlbanyNY), 13(24), 25931-25943. DOI
- Pollak, N., Dölle, C., Ziegler, M. (2007) The power to reduce: pyridinenucleotides--small molecules with a multitude of functions. Biochem. J., 402(2),205-18. DOI
- Sirota, T.V. (2020) Effect of sulfur-containing compounds on the quinoidprocess of adrenaline autoxidation; Potential Neuroprotectors. BiomeditsinskayaKhimiya, 65(4), 316-323. DOI