A Simple Technique for Isolating Plasmids From Escherichia Coli for Efficient Chemical Transfection of Human Cell Culture
Federal Research and Clinical Center of Physical-Chemical Medicine of Federal Medical Biological Agency,
1a Malaya Pirogovskaya str., Moscow, 119435 Russia; *e-mail: lazarev@rcpcm.org
Keywords: bacterial plasmids; transfection; human cell line
DOI:10.18097/BMCRM00170
Currently, a large number of reagent kits are commercially available for the isolation of highly purified plasmid DNA for subsequent transfection of human cell lines. However, due to high cost and logistical problems, it may be necessary to isolate plasmid DNA using only the simplest reagents and materials. We present one of the possible methods for such DNA isolation, suitable for routine laboratory use. It is based on well-known principles and methods for plasmid DNA purification, has minimal cost, does not require special skills, and is easily scalable. The technique includes the steps of alkaline lysis, purification with silica particles and gel filtration. It was shown that plasmids isolated using the proposed method transfect human embryonic kidney Expi293F cells no less efficiently than plasmids purified using a specialized Qiagen plasmid maxi kit («Qiagen», USA).
|
CLOSE

|
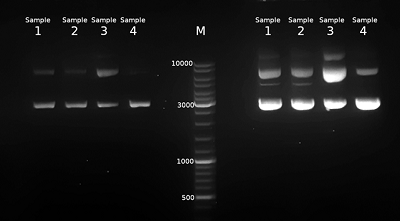
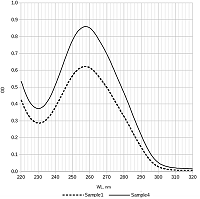
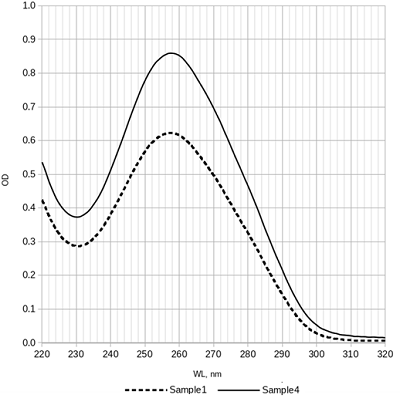
Table 1.
Results of pDNA isolation. Isolation steps: AL—alkaline lysis, silica—binding with silicon dioxide, GF—gel filtration, IPA—isopropanol precipitation, Qiagen kit—Qiagen plasmid maxi kit. OD260/OD280 is the ratio of optical densities of DNA solutions at 260 nm and 280 nm.
|
|
CLOSE

|
Table 2.
Efficiency of transfection of Expi293F cells with plasmid samples isolated by different methods.
|
FUNDING
This research was supported by the State Assignment on the Development of a complex therapy scheme for drug-resistant pathogens of infectious diseases using bacteriophages or their de-rivatives in combination with antibacterial drugs (Code: Bacteriophage-2) (Russia).
REFERENCES
- Kim T.K., Eberwine J.H. (2010) Mammalian cell transfection: the present and the future. Analytical and Bioanalytical Chemistry, 397(8), 3173-3178. DOI
- Fus-Kujawa A., Prus P., Bajdak-Rusinek K., Teper P., Gawron K., Kowalczuk A., Sieron A.L. (2021) An Overview of Methods and Tools for Transfection of Eukaryotic Cells in vitro. Frontiers in Bioengineering and Biotechnology, 9, 701031. DOI
- Butash K.A., Natarajan P., Young A., Fox D.K. (2000) Reexamination of the effect of endotoxin on cell proliferation and transfection efficiency. Biotechniques, 29(3), 610-14. DOI
- Remaut K., Sanders N.N., Fayazpour F., Demeester J., De Smedt S.C. (2006) Influence of plasmid DNA topology on the transfection properties of DOTAP/DOPE lipoplexes. Journal of Controlled Release. 115(3), 335-343. DOI
- Birnboim H.C., Doly J. (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Research, 7(6), 1513-1523. DOI
- Figueroa-Bossi N., Balbontin R., Bossi L. (2022) Preparing Plasmid DNA from Bacteria. Cold Spring Harbor protocols, Aug 5, online ahead of print. DOI
- Rinaldi M., Fioretti D., Iurescia S. (2014) DNA Vaccines (Vol. 1143). Springer New York. DOI