New Highly Sensitive Methods for Electroanalysis of the Catalytic Activity of Enzymes of Medical Significance
1Institute of Biomedical Chemistry, 10 Pogodinskaya str., Moscow, 119121 Russia; *e-mail: viktoria.shumyantseva@ibmc.msk.ru
2Department of Biochemistry, Pirogov Russian National Research Medical University, 1 Ostrovitianova str., Moscow, 117997 Russia
Keywords: cytochrome P450; substrates; inhibitors; asparaginase; enzymatic catalysis; electrochemical analysis
DOI:10.18097/BMCRM00225
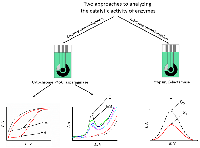
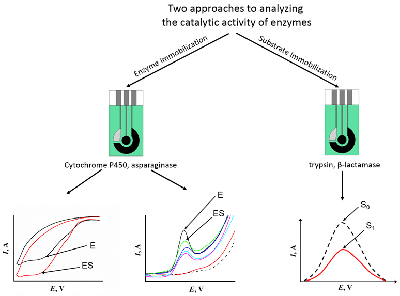
The review is devoted to new highly effective methods for analyzing the catalytic activity of enzymes of medical significance, such as cytochromes P450, trypsin, asparaginase, beta-lactamase, and nucleases. The methods are based on registration the specific activity of enzymes using electroanalytical methods. The review analyzes the experimental data obtained by the authors. Two platforms have been developed that allow quantitative measurement of catalytic activity based on the electrochemical properties of the enzyme (cytochrome P450, bactosomes, asparaginase) or substrate (trypsin, nucleases, restriction enzymes, beta-lactamase).
|
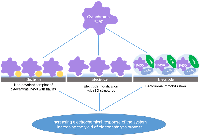
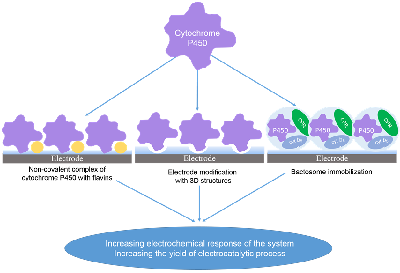
Figure 4.
Methods for increasing the catalytic activity of cytochromes P450 in electrochemical systems.
|
|
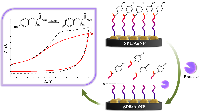
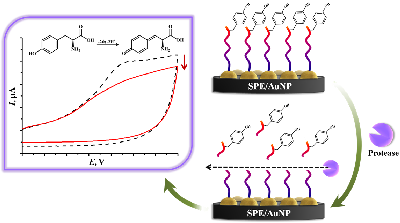
Figure 5.
Algorithm for electroanalysis of proteases based on the hydrolysis of peptides containing the electroactive amino acid tyrosine.
|
|
CLOSE

|
Table 1.
Determination of cytochrome P450 activity by electrochemical analysis of reaction products.
|
FUNDING
The work was performed within the framework of the Program for Basic Research in the Russian Federation for a long-term period (2021-2030) (№122030100168-2).
REFERENCES
- Shajhutdinova, Z., Pashirova, T., Masson, P. (2022) Kinetic processes in enzymatic nanoreactors for in vivo detoxification. Biomedicines, 10(4), 784. DOI
- Pashirova, T., Shaihutdinova, Z., Mansurova, M., Kazakova, R., Shambazova, D., Bogdanov, A., Tatarinov, D., Daudé, D., Jacquet, P., Chabrièûre, E., Masson, P. (2022) Enzyme nanoreactor for in vivo detoxification of organophosphates. ACS Appl. Mater. Interfaces, 14(17), 19241–19252. DOI
- Wohlgemuth, R. (2021) Biocatalysis — key enabling tools from biocatalytic one-step and multi-step reactions to biocatalytic total synthesis. New Biotechnol., 60, 113–123. DOI
- Singh, R.S., Singh, T., Singh, A.K. (2019) Enzymes as Diagnostic Tools. In: Advances in Enzyme Technology (Singh, R.S., Singhania, R.R., Pandey, A., Larroche, C., eds.), Elsevier, Netherlands, pp. 225–271. DOI
- Zhao, W.W., Xu, J.J., Chen, H.Y. (2017) Photoelectrochemical enzymatic biosensors. Biosens. Bioelectron., 92, 294–304. DOI
- Klyushova, L.S., Perepechaeva, M.L., Grishanova, A.Y. (2022) The role of CYP3A in health and disease. Biomedicines, 10(11), 2686. DOI
- Li, Z., Jiang, Y., Guengerich, F.P., Ma, L., Li, S., Zhang, W. (2020) Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications. J. Biol. Chem., 295(3), 833–849. DOI
- di Nardo, G., Gilardi, G. (2020) Natural compounds as pharmaceuticals: The key role of cytochromes P450 reactivity. Trends Biochem. Sci., 45(6), 511–525, DOI: 10.1016/j.tibs.2020.03.004.
- Krishnan, S. (2020) Bioelectrodes for evaluating molecular therapeutic and toxicity properties. Curr. Opin. Electrochem., 19, 20–26. DOI
- Hrycay, E.G., Bandiera, S.M. (2012) The monooxygenase, peroxidase, and peroxygenase properties of cytochrome P450. Arch. Biochem. Biophys., 522(2), 71–89. DOI
- Guengerich, F.P. (2021) Drug Metabolism: Cytochrome P450. In: Reference Module in Biomedical Sciences. Elsevier, Netherlands. DOI
- Mi, L., Wang, Z., Yang, W., Huan, C., Zhou, B., Hu, Y., Liu, S. (2023) Cytochromes P450 in biosensing and biosynthesis applications: Recent progress and future perspectives. TrAC, Trends Anal. Chem., 158, 116791. DOI
- Schneider, E., Clark, D.S. (2013) Cytochrome P450 (CYP) enzymes and the development of CYP biosensors. Biosens. Bioelectron., 39, 1–13. DOI
- Sun, X., Sun, J., Ye, Y., Ji, J., Sheng, L., Yang, D., Sun, X. (2023) Metabolic pathway-based self-assembled Au@MXene liver microsome electrochemical biosensor for rapid screening of aflatoxin B1. Bioelectrochemistry, 151, 108378. DOI
- Shumyantseva, V.V., Kuzikov, A.V., Masamrekh, R.A., Bulko, T.V., Archakov, A.I. (2018) From electrochemistry to enzyme kinetics of cytochrome P450. Biosens. Bioelectron., 15, 192–204. DOI
- Kumar, N., He, J. Rusling, J.F. (2023) Electrochemical transformations catalyzed by cytochrome P450s and peroxidases. Chem. Soc. Rev., 52, 5135–5171. DOI
- Kostin, V.A., Zolottsev, V.A., Kuzikov, A.V., Masamrekh, R.A., Shumyantseva, V.V., Veselovsky, A.V., Stulov, S.V., Novikov, R.A., Timofeev, V.P., Misharin, A.Y. (2016) Oxazolinyl derivatives of [17 (20) E]-21-norpregnene differing in the structure of A and B rings. Facile synthesis and inhibition of CYP17A1 catalytic activity. Steroids, 115, 114–122. DOI
- Archakov, A.I., Lisitsa, A.V., Ipatova, O.M., Misharin, A.Yu., Stulov, S.V., Shishova, D.K., Shumyantseva, V.V., Kuzikov, A.V., Veselovsky, A.V. (2017) Pregn-17(20)-ene derivatives with antitumor activity, Russian State Patent Agency Certificate, No. 2617698 of 26.04.2017.
- Makhova, A.A., Shumyantseva, V.V., Shikh, E.V., Butko, T.V., Suprun, E.V., Kuzikov, A.V., Kukes, V.G., Archakov, A.I. (2013) Regulation of the activity of drug metabolism enzymes — cytochromes P450 3A4 and 2C9 by biologically active compounds. Molekulyarnaya Meditsina, 5, 49-53.
- Shumyantseva, V.V., Bulko, T.V., Suprun, E.V., Kuzikov, A.V., Agafonova, L.E., Archakov, A.I. (2015) Electrochemical methods for biomedical investigations. Biomeditsinskaya Khimiya, 61(2), 188–202. DOI
- Shumyantseva, V.V., Makhova, A.A., Bulko, T.V., Kuzikov, A.V., Shich, E.V., Kukes, V.G., Archakov, A.I. (2015) Electrocatalytic cycle of P450 cytochromes: The protective and stimulating roles of antioxidants. RSC Adv., 5(87), 71306–71313.
- Shih, E.V., Fomin, Е.V., Shumyantseva, V.V., Bulko, T.V. (2012) Combined therapy of elderly patients with regard to drugs metabolism. Klinicheskaya Gerontologiya, 18(3–4), 54–58.
- Makhova, A.A., Shikh, E.V., Bulko, T.V., Sizova, Z.M., Shumyantseva, V.V. (2019) The influence of taurine and L-carnitine on 6 β-hydroxycortisol/cortisol ratio in human urine of healthy volunteers. Drug Metab. Pers. Ther., 34(3), 20190013. DOI
- Makhova, A.A., Shikh, E.V., Bulko, T.V., Komissarenko, I.A., Sizova, Zh.M., Shumyantseva, V.V. (2020) Effects of taurine and L-carnitine on the activity of cytochrome P450 3A4 in volunteers. Experimentalnaya i Klinicheskaya Farmakologiya, 83(2), 34–37. DOI
- Koroleva, P.I., Kuzikov, A.V., Masamrekh, R.A., Filimonov, D.A., Dmitriev, A.V., Zaviyalova, M.G., Rikova, S.M., Shich, E.V., Makhova, A.A., Bulko, T.V., Gilep, A.A., Shumyantseva, V.V. (2020) Modeling of drug-drug interactions between omeprazole and erythromycin in the cytochrome P450-dependent system in vitro. Biomeditsinskaya Khimiya, 66(3), 241–249. DOI
- Shumyantseva, V.V., Koroleva, P.I., Bulko, T.V., Sergeev, G.V., Usanov, S.A. (2022) Predicting drug-drug interactions by electrochemically driven cytochrome P450 3A4 reactions. Drug Metab. Pers. Ther., 37(3), 241–248. DOI
- Masamrekh, R.A., Kuzikov, A.V., Shcherbakov, K.A., Veselovsky, A.V., Filimonov, D.A., Dmitriev, A.V., Zavialova, M.G., Archakov, A.I., Shumyantseva, V.V., Haurychenka, Y.I., Gilep, A.A., Shkel, T.V., Strushkevich, N.V., Usanov, S.A. (2020) In vitro interactions of abiraterone, erythromycin, and CYP3A4: Implications for drug-drug interactions. Fundam. Clin. Pharmacol., 34(1), 120–130. DOI
- Masamrekh, R.A., Kuzikov, A.V., Filippova, T.A., Sherbakov, K.A., Veselovsky, A.V., Shumyantseva, V.V. (2022) The interactions of abiraterone and its pharmacologically active metabolite D4A with cytochrome P450 2C9 (CYP2C9). Biomeditsinskaya Khimiya, 68(3), 201–211. DOI
- Shumyantseva, V.V., Bulko, T.V., Koroleva, P.I., Shikh, E.V., Makhova, A.A., Kisel, M.S., Haidukevich, I.V., Gilep, A.A. (2022) Human cytochrome P450 2C9 and its polymorphic modifications: Electroanalysis, catalytic properties, and approaches to the regulation of enzymatic activity. Processes, 10(2), 383. DOI
- Shumyantseva, V.V., Bulko, T.V., Kuzikov, A.V., Masamrekh, R.A., Konyakhina, A.Y., Romanenko, I., Max, J.B., Köhler, M., Gilep, A.A., Usanov, S.A., Pergushov, D.V., Schacher, F.H., Sigolaeva, L.V. (2020) All-electrochemical nanocomposite two-electrode setup for quantification of drugs and study of their electrocatalytical conversion by cytochromes P450. Electrochim. Acta, 336, 135579. DOI
- Kuzikov, A.V., Filippova, T.A., Masamrekh, R.A. Shumyantseva, V.V. (2022) Electrochemical determination of (S)-7-hydroxywarfarin for analysis of CYP2C9 catalytic activity. J. Electroanal. Chem., 904, 115937. DOI
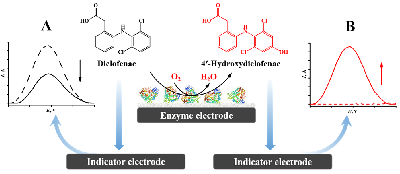
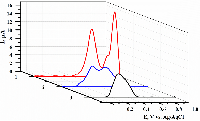
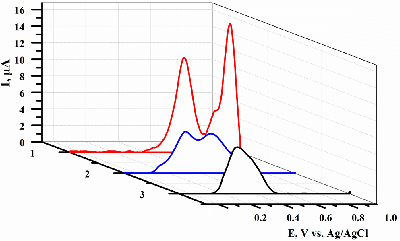
- Kuzikov, A.V., Filippova, T.A., Masamrekh, R.A., Shumyantseva, V.V. (2022) Electroanalysis of 4′-hydroxydiclofenac for CYP2C9 enzymatic assay. Electrocatalysis, 13(5), 630–640. DOI
- Filippova, T.A., Masamrekh, R.A., Shumyantseva, V.V., Khudoklinova, Y.Y., Kuzikov, A.V. (2023) Voltammetric analysis of (S)-O-desmethylnaproxen for determination of CYP2C9 demethylase activity. BioNanoScience, 13(3), 1278–1288. DOI
- Kuzikov, A.V., Filippova, T.A., Masamrekh, R.A., Shumyantseva, V.V. (2022) Biotransformation of phenytoin in the electrochemically-driven CYP2C19 system. Biophys. Chem., 291, 106894. DOI
- Kuzikov, A.V., Masamrekh, R.A., Filippova, T.A., Tumilovich, A.M., Strushkevich, N.V., Gilep, A.A., Khudoklinova, Y.Y., Shumyantseva, V.V. (2024) Bielectrode strategy for determination of CYP2E1 catalytic activity: Electrodes with bactosomes and voltammetric determination of 6-hydroxychlorzoxazone. Biomedicines, 12(1), 152. DOI
- Kuzikov, A.V., Masamrekh, R.A., Filippova, T.A., Haurychenka, Y.I., Gilep, A.A., Shkel, T.V., Strushkevich, N.V., Usanov, S.A., Shumyantseva, V.V. (2020) Electrochemical oxidation of estrogens as a method for CYP19A1 (aromatase) electrocatalytic activity determination. Electrochim. Acta, 333, 135539. DOI
- Tracy, T.S. (2006) Atypical cytochrome P450 kinetics: Implications for drug discovery. Drugs in R&D, 7, 349–363. DOI
- Alim, S., Vejayan, J., Yusoff, M.M., Kafi, A.K.M. (2018) Recent uses of carbon nanotubes & gold nanoparticles in electrochemistry with application in biosensing: A review. Biosens. Bioelectron., 121, 125–136. DOI
- Shumyantseva, V.V., Kuzikov, A.V., Masamrekh, R.A., Filippova, T.A., Koroleva, P.I., Agafonova, L.E., Bulko, T.V., Archakov, A.I. (2022) Enzymology on an electrode and in a nanopore: Analysis algorithms, enzyme kinetics, and perspectives. BioNanoScience, 12, 1341–1355. DOI
- Shumyantseva, V.V., Koroleva, P.I., Bulko, T.V., Shkel, T.V., Gilep, A.A., Veselovsky, A.V. (2023) Approaches for increasing the electrocatalitic efficiency of cytochrome P450 3A4. Bioelectrochemistry, 149, 108277. DOI
- Shumyantseva, V.V., Koroleva, P.I., Gilep, A.A., Napolskii, K.S., Ivanov, Yu.D., Kanashenko, S.L., Archakov, A.I. (2022) Increasing the efficiency of cytochrome P450 3A4 electrocatalysis using electrode modification with spatially ordered anodic aluminum oxide-based nanostructures for investigation of metabolic transformations of drugs. Dokl. Biochem. Biophys., 506, 215–219. DOI
- Koroleva, P.I., Bulko, T.V., Agafonova, L.E., Shumyantseva, V.V. (2023) Catalytic and electrocatalytic mechanisms of cytochromes P450 in the development of biosensors and bioreactors. Biochemistry (Moscow), 88(10), 1645–1657. DOI
- Koroleva, P.I., Gilep, A.A., Kraevsky, S.V., Tsybruk, T.V., Shumyantseva, V.V. (2023) Improving the efficiency of electrocatalysis of cytochrome P450 3A4 by modifying the electrode with membrane protein streptolysin O for studying the metabolic transformations of drugs. Biosensors, 13(4), 457. DOI
- Nerimetla, R., Premaratne, G., Liu, H., Krishnan, S. (2018) Improved electrocatalytic metabolite production and drug biosensing by human liver microsomes immobilized on amine-functionalized magnetic nanoparticles. Electrochim. Acta, 280, 101–107. DOI
- Walker, A., Walgama, C., Nerimetla, R., Alavi, S.H., Echeverria, E., Harimkar, S.P., McIlroy, D.N., Krishnan, S. (2020) Roughened graphite biointerfaced with P450 liver microsomes: Surface and electrochemical characterizations. Colloids Surf., B, 189, 110790. DOI
- Vimal, A., Kumar, A. (2017) Biotechnological production and practical application of L-asparaginase enzyme. Biotechnol. Genet. Eng. Rev., 33(1), 40–61. DOI
- Pokrovskaya, M.V., Pokrovsky, V.S., Aleksandrova, S.S., Sokolov, N.N., Zhdanov, D.D. (2022) Molecular analysis of L-asparaginases for clarification of the mechanism of action and optimization of pharmacological functions. Pharmaceutics, 14, 599. DOI
- Darvishi, F., Jahanafrooz, Z., Mokhtarzadeh, A. (2022) Microbial L-asparaginase as a promising enzyme for treatment of various 516 cancers. Appl. Microbiol. Biotechnol., 106, 5335–5347. DOI
- Shumyantseva, V., Bulko, T., Pronina, V., Kanashenko, S., Pokrovskaya, M., Aleksandrova, S., Zhdanov, D. (2022) Electroenzymatic model system for the determination of catalytic activity of Erwinia carotovora L-asparaginase. Processes, 10(7), 1313. DOI
- Ćwilichowska, N., Świderska, K.W., Dobrzyń, A., Drąg, M., Poręba, M. (2022) Diagnostic and therapeutic potential of protease inhibition. Mol. Aspects Med., 88, 101144. DOI
- Hua, Y., Nair, S. (2015) Proteases in cardiometabolic diseases: Pathophysiology, molecular mechanisms and clinical applications. Biochim. Biophys. Acta Mol. Basis Dis., 1852(2), 195–208. DOI
- Craik, C.S., Page, M.J., Madison, E.L. (2011) Proteases as therapeutics. Biochem. J., 435(1), 1–16. DOI
- Hotinger, J.A., Gallagher, A.H., May, A.E. (2022) Phage-related ribosomal proteases (Prps): Discovery, bioinformatics, and structural analysis. Antibiotics, 11(8), 1109. DOI
- Anirudhan, V., Lee, H., Cheng, H., Cooper, L., Rong, L. (2021) Targeting SARS-CoV-2 viral proteases as a therapeutic strategy to treat COVID-19. J. Med. Virol., 93(5), 2722–2734. DOI
- Filippova, T.A., Masamrekh, R.A., Khudoklinova, Y.Y., Shumyantseva, V.V., Kuzikov, A.V. (2024) The multifaceted role of proteases and modern analytical methods for investigation of their catalytic activity. Biochimie, 222, 169–194. DOI
- Fabry, P., Moutet, J.C. (2013) Sensitivity and Selectivity of Electrochemical Sensors. In: Chemical and Biological Microsensors: Applications in Liquid Media (Fouletier, J., Fabry, P., eds.), ISTE ltd, London – John Wiley & Sons, Inc, Hoboken, NJ, pp. 45-80. DOI
- Ostojić, J., Herenda, S., Bešić, Z., Miloš, M., Galić, B. (2017) Advantages of an electrochemical method compared to the spectrophotometric kinetic study of peroxidase inhibition by boroxine derivative. Molecules, 22(7), 1120. DOI
- Rodriguez-Rios, M., Megia-Fernandez, A., Norman, D.J., Bradley, M. (2022) Peptide probes for proteases — innovations and applications for monitoring proteolytic activity. Chem. Soc. Rev., 51(6), 2081–2120. DOI
- González-Fernández, E., Avlonitis, N., Murray, A.F., Mount, A.R., Bradley, M. (2016) Methylene blue not ferrocene: Optimal reporters for electrochemical detection of protease activity. Biosens. Bioelectron., 84, 82–88. DOI
- Zambry, N.S., Obande, G.A., Khalid, M.F., Bustami, Y., Hamzah, H.H., Awang, M.S., Aziah, I., Manaf, A.A. (2022) Utilizing electrochemical-based sensing approaches for the detection of SARS-CoV-2 in clinical samples: A review. Biosensors, 12(7), 473. DOI
- Filippova, T.A., Masamrekh, R.A., Shumyantseva, V.V., Latsis, I.A., Farafonova, T.E., Ilina, I.Y., Kanashenko, S.L., Moshkovskii, S.A., Kuzikov, A.V. (2023) Electrochemical biosensor for trypsin activity assay based on cleavage of immobilized tyrosine-containing peptide. Talanta, 257, 124341. DOI
- Anne, A., Chovin, A., Demaille, C. (2012) Optimizing electrode-attached redox-peptide systems for kinetic characterization of protease action on immobilized substrates. Observation of dissimilar behavior of trypsin and thrombin enzymes. Langmuir, 28(23), 8804–8813. DOI
- Ucar, A., González-Fernández, E., Staderini, M., Avlonitis, N., Murray, A.F., Bradley, M., Mount, A.R. (2020) Miniaturisation of a peptide-based electrochemical protease activity sensor using platinum microelectrodes. Analyst, 145(3), 975–982. DOI
- Li, R., Chen, X., Zhou, C., Dai, Q.Q., Yang, L. (2022) Recent advances in β-lactamase inhibitor chemotypes and inhibition modes. Eur. J. Med. Chem., 242, 114677. DOI
- di Felice, F., Micheli, G., Camilloni, G. (2019) Restriction enzymes and their use in molecular biology: An overview. J. Biosci., 44(2), 38. DOI
- Gonzalez, J.G., Hernandez, F.J. (2022) Nuclease activity: An exploitable biomarker in bacterial infections. Expert Rev. Mol. Diagn., 22(3), 265–294. DOI
- Ding, J., Qin, W. (2013) Potentiometric sensing of nuclease activities and oxidative damage of single-stranded DNA using a polycation-sensitive membrane electrode. Biosens. Bioelectron., 47, 559–565. DOI
- Agafonova, L.E., Zhdanov, D.D., Gladilina, Y.A., Shishparenok, A.N., Shumyantseva, V.V. (2024) Electrochemical approach for the analysis of DNA degradation in native DNA and apoptotic cells. Heliyon, 10(3), e25602. DOI