Comparison of the Results of SPR Analysis of Antigen-Antibody Interactions Performed Using Optical Biosensors Biacore X-100 (“Cytiva”, USA) and MI-S200D (“Inter-Bio”, China)
Institute of Biomedical Chemistry, 10 Pogodinskaya str., Moscow, 119121 Russia; *gnedenko.oksana@gmail.com
Keywords: surface plasmon resonance (SPR); optical biosensors; antigen-antibody interaction; rate constants of assoсiation and dissociation of the complex (kon, koff); equilibrium dissociation constant (Kd)
DOI:10.18097/BMCRM00246
Limited availability of Western scientific equipment, explains a growing need to switch to using scientific instruments made in China. This is especially true in the case of optical biosensors operating on the surface plasmon resonance (SPR) effect. However, comparability of experimental data obtained using Western and Chinese biosensors has not been investigated yet. In this work we have comparedresults of SPR analysis of the kinetics and affinity of interaction of IgG2a and IgG1 antibodies with protein A. Two biosensos Biacore X-100 (“Cytiva”, USA) and MI-S200D (“Inter-Bio”, China) have been used. It was shown that the values of the association rate constants obtained on both devices for two antibodies were close within one order of magnitude. For IgG1 antibodies, the dissociation rate constants were almost identical. Both devices provide high-quality data that are well described by a simple 1:1 model (Langmuir binding).


|
Figure 2.
The structure of Biacore CM5 optical chip. A - a chip storage case (left) and an optical chip (right). B - reverse side.
|


|
Figure 4.
Schema of flow optical biosensors operating on the SPR effect [12]. The frames highlight the replaceable parts (optical chips): Biacore CM5 (solid line) and IB-CM5 (dashed line).
|
|
CLOSE

|
Table 1.
Comparison of parameters of Biacore X-100 and MI-S200D biosensors.
|
|
CLOSE

|
Table 2
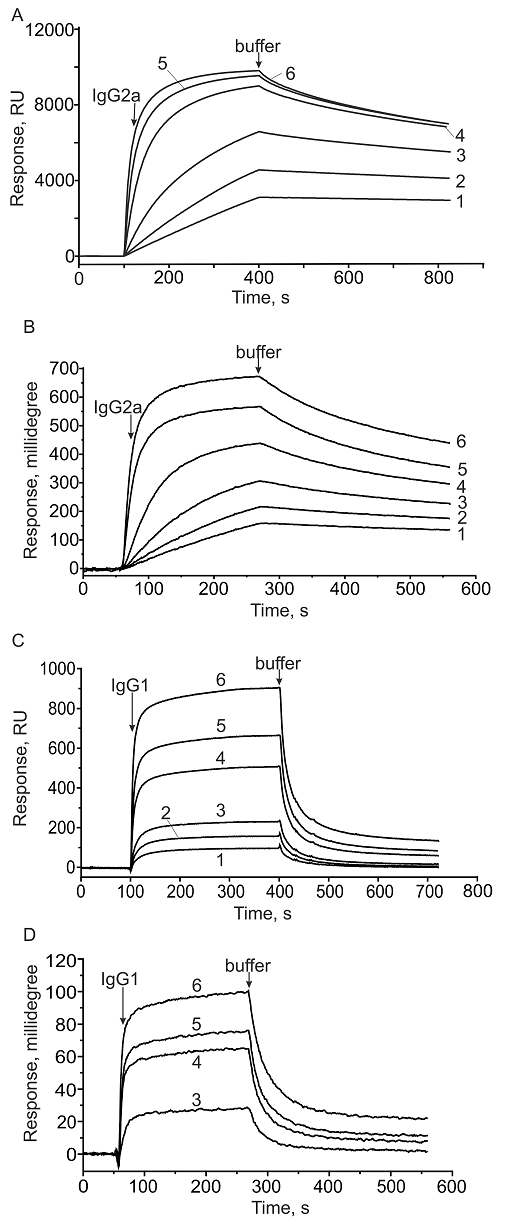
Kinetic parameters (kon and koff) and equilibrium dissociation constant Kd of antibody complexes with immobilized protein A.
|
FUNDING
The work was carried out within the framework of the Program for Basic Research in the Russian Federation for a long-term period (2021–2030) (No. 122030100168-2).
REFERENCES
- Myszka, D.G. (2000) Kinetic, equilibrium, and thermodynamic analysis of macromolecular interactions with BIACORE. В Methods in Enzymology (Т. 323, pp. 325–340). Elsevier. DOI
- Gnedenko, O.V., Mezentsev, Y.V., Molnar, A.A., Lisitsa, A.V., Ivanov, A.S., Archakov, A.I. (2013) Highly sensitive detection of human cardiac myoglobin using a reverse sandwich immunoassay with a gold nanoparticle-enhanced surface plasmon resonance biosensor. Analytica Chimica Acta, 759, 105–109. DOI
- Davidoff, S.N., Ditto, N.T., Brooks, A.E., Eckman, J., Brooks, B.D. (2015) Surface plasmon resonance for therapeutic antibody characterization. Fang, В.Y.(Ред.), Label-Free Biosensor Methods in Drug Discovery (pp. 35–76). New York, NY: Springer New York. DOI
- Regenmortel, M.H.V.V., Altschuh, D., Chatellier, J., Christensen, L., Rauffer-Bruyère, N., Richalet-Secordel, P., Witz, J., Zeder-Lutz, G. (1998) Measurement of antigen–antibody interactions with biosensors. Journal of Molecular Recognition, 11(1–6), 163–167. DOI
- Myszka, D.G. (1997) Kinetic analysis of macromolecular interactions using surface plasmon resonance biosensors. Current Opinion in Biotechnology, 8(1), 50–57. DOI
- McDonnell, J.M. (2001) Surface plasmon resonance: towards an understanding of the mechanisms of biological molecular recognition. Current Opinion in Chemical Biology, 5(5), 572–577. DOI
- Cooper, M.A. (2002) Optical biosensors in drug discovery. Nature Reviews Drug Discovery, 1(7), 515–528. DOI
- Knowling, S., Clark, J., Sjuts, H., Abdiche, Y.N. (2020) Direct comparison of label-free biosensor binding kinetics obtained on the Biacore 8K and the Carterra LSA. SLAS Discovery, 25(9), 977–984. DOI
- Yang, D., Singh, A., Wu, H., Kroe-Barrett, R. (2016) Comparison of biosensor platforms in the evaluation of high affinity antibody-antigen binding kinetics. Analytical Biochemistry, 508, 78–96. DOI
- Bright, R.A., Carter, D.M., Crevar, C.J., Toapanta, F.R., Steckbeck, J.D., Cole, K.S., Kumar, N.M., Pushko, P., Smith, G., Tumpey, T.M., Ross, T.M. (2008) Cross-Clade Protective Immune Responses to Influenza Viruses with H5N1 HA and NA Elicited by an Influenza Virus-Like Particle. PLoS ONE, 3(1), e1501. DOI
- Fägerstam, L.G., Frostell-Karlsson, Å., Karlsson, R., Persson, B., Rönnberg, I. (1992) Biospecific interaction analysis using surface plasmon resonance detection applied to kinetic, binding site and concentration analysis. Journal of Chromatography A, 597(1–2), 397–410. DOI
- Schuck, P. (1997) Use of surface plasmon resonance to probe the equilibrium and dynamic aspects of interactions between biological macromolecules. Annual Review of Biophysics and Biomolecular Structure, 26, 541–566. DOI
- Hober, S., Nord, K., Linhult, M. (2007) Protein A chromatography for antibody purification. Journal of Chromatography B, 848(1), 40–47. DOI