DETECTION OF THE KIDNEY TUMOR SUPPRESSOR DUSP9 IN THE URINE OF HEALTHY DONORS
Granov Russian Research Center of Radiology and Surgical Technologies, 70 Leningradskaya St., Pesochnyy, Saint-Petersburg 197758, Russia; *e-mail: polischouka@mail.ru
Keywords: DUSP9/MKP-4 protein phosphatase; renal carcinoma; extracellular vesicles, exosomes
DOI:10.18097/BMCRM00267
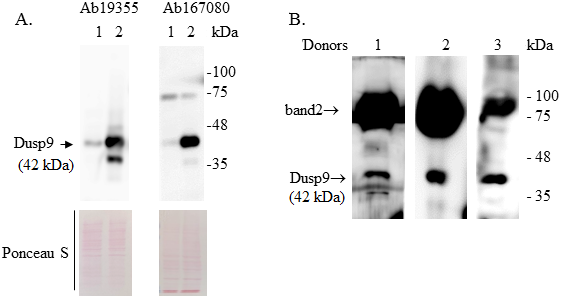
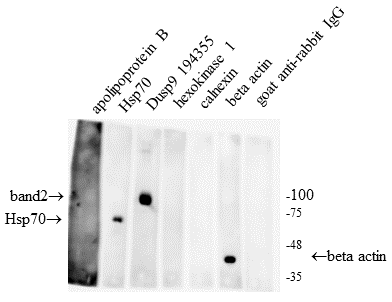
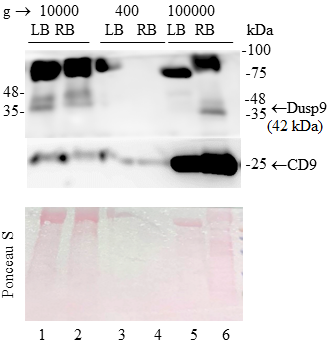
The DUSP9/MKP-4 belongs to the family of bispecific protein phosphatases that negatively regulate MAP kinases (ERK, p38 and JNK). The expression of DUSP9 is significantly (20-80-fold) reduced compared to normal tissue in 95% of the studied human renal cell carcinoma samples. These and other scientific data indicate that DUSP9 is an attractive target for the use in clinical practice. However, so far, postoperative tumor biopsy samples remain the only source of clinical material in which expression of DUSP9 has been studied.. This significantly limits the possibilities of using DUSP9 for clinical purposes. The purpose of this work was to find out whether it would be possible to detect DUSP9 in human urine. In the study we used human kidney carcinoma ACHN cells transfected with a vector, expressing DUSP9 and urine samples from 3 healthy volunteers. DUSP9 protein was detected by the Western blot method in precipitates obtained by centrifugation. We have shown that the DUSP9 protein is present in the pellet of the urine fraction obtained by low-speed centrifugation (10000 g). It is also present in the fraction of extracellular vesicles enriched with exosomes. The obtained result indicates that the analysis of DUSP9 expression is possible using a liquid biopsy, which, in turn, can significantly expand the applicability of this analysis for clinical purposes.
|
CLOSE

|
Table 1.
Characteristics of the antibodies used in the study
|
FUNDING
This work was supported by an assignment of Ministry of Health of Russia №124021600040-2.
REFERENCES
- Yakubovich, E.I., Polischouk, A.G., Evtushenko, V.I. (2023) Exogenousexpression of protein phosphatase DUSP9 reduces the rate of migrationof kidney carcinoma cells. Voprosy Onkologii, 69(3), 429–436. DOI
- Khoubai, F.Z., Grosset, C.F. (2021) DUSP9, a dual-specificity phosphatasewith a key role in cell biology and human diseases. International Journal ofMolecular Sciences, 22(21), 11538. DOI
- Granov, A.M., Vershinina, S.F., Yakubovich, E.I., Markochev, A.B.,Yevtushenko, V.I. (2016) The antitumor effect of expression plasmid bearingDUSP9 gene in SHR mice having solid ehrlich carcinoma. MedicinskijAkadimicheskij Zhurnal, 16(3), 75–81.
- Yakubovich, E.I., Polischouk, A.G., Evtushenko, V.I. (2020) Principlesand problems of exosome isolation from biological fluids. Biochemistry(Moscow), Supplement Series A: Membrane and Cell Biology, 16(2), 115-126. DOI
- Fernández-Llama, P., Khositseth, S., Gonzales, P.A., Star, R.A., Pisitkun, T.,Knepper, M.A. (2010) Tamm-Horsfall protein and urinary exosome isolation.Kidney International, 77(8), 736–742. DOI
- Chen, H.F., Chuang, H.C., Tan, T.H. (2019) Regulation of dual-specificityphosphatase (DUSP) ubiquitination and protein stability. International Journalof Molecular Sciences, 20(11), 2668. DOI
- Tenchov, R., Sasso, J.M., Wang, X., Liaw, W.S., Chen, C.A., Zhou, Q.A. (2022)Exosomes - nature’s lipid nanoparticles, a rising star in drug delivery anddiagnostics. ACS Nano, 16(11), 17802-17846. DOI