Recombinant Proteins Combining the Inter-Domain Region of Pneumococcal Surface Antigen "A" and Adaptive W-Type Polypeptide of Thermotoga Bacteria as a Potential Component Base for the Development of New Diagnostics and Genetically Engineered Subunit Vaccines against Pneumococcal Infection
1Institute of Biomedical Chemistry, 10 Pogodinskaya str., Moscow, 119121 Russia; *e-mail: molbiol_ibm@inbox.ru
2I. Mechnikov Research Institute of Vaccines and Sera, 5А Malyi Kazennyi lane, Moscow, 105064 Russia
Keywords: fusion proteins; pneumococcal surface protein; thermostable protein; vaccine-valuable protein; Escherichia coli; heterologous expression
DOI:10.18097/BMCRM00237
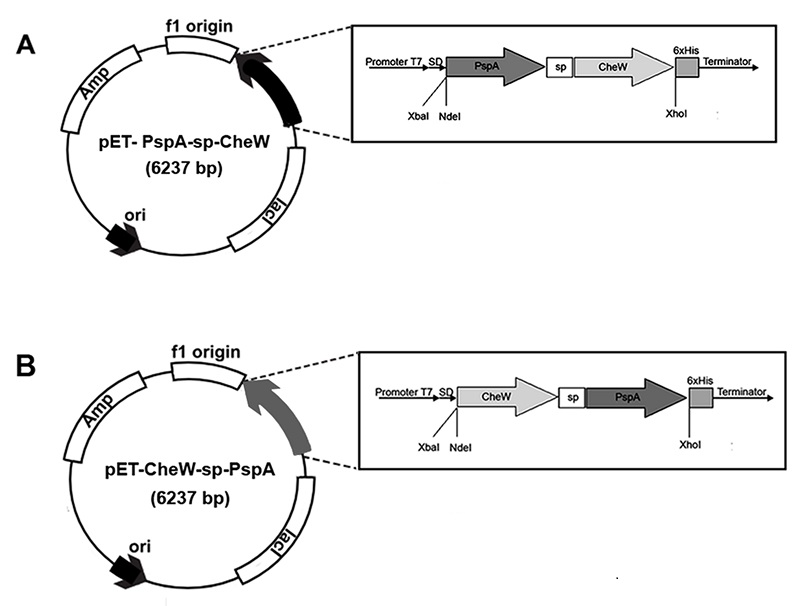
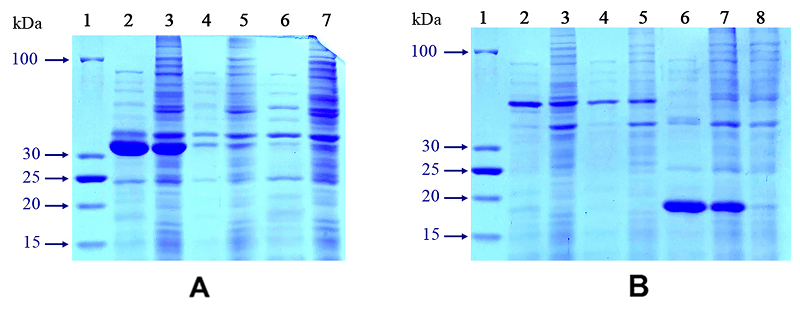
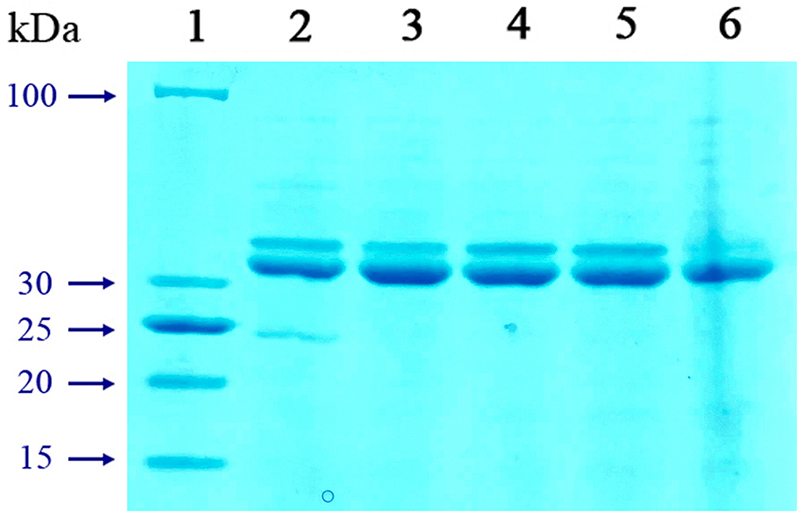
The problems related to the development of pneumococcal vaccines require combination of traditional solutions and alternative systems optimizing this procedure. Recombinant subunit vaccines have undeniable advantages over inactivated and live-attenuated vaccines: they induce cell-mediated and humoral immunologic responses effectively and with high specificity, but without the risks associated with authentic pathogen processing. However, subunit vaccines require specific adjuvants to enhance the immune response or special fusion partners to improve solubility, expression and optimize subsequent fine purification of the protein of interest. In the framework of this work, a structurally conserved region of the most immunogenic region of the vaccine-valuable surface antigen PspA of Streptococcus pneumoniae was chosen as a model protein, and the adaptive polypeptide CheW from the hyperthermophilic microorganism Thermotoga petrophila was used as a promising fusion protein. Appropriate expression plasmid vectors were designed in silico and constructed in vitro. Efficient E. coli producer strains were obtained and appropriate conditions for heterologous production of chimeric proteins were selected. The fusion partner from T. petrophila positively influenced the properties of the resulting constructs such as thermostability, solubility, and homogeneity. During this work, the optimal pH and temperature ranges of the created proteins were determined, and the principles of low-stage purification were elaborated. We obtained and characterized new proteins, which were not previously found in nature in a similar bioconfiguration. The results indicate that the biotechnologically valuable characteristics of the fusion protein were more expressed when the adaptive CheW protein was combined with the N-terminus of the PspA antigen.

|
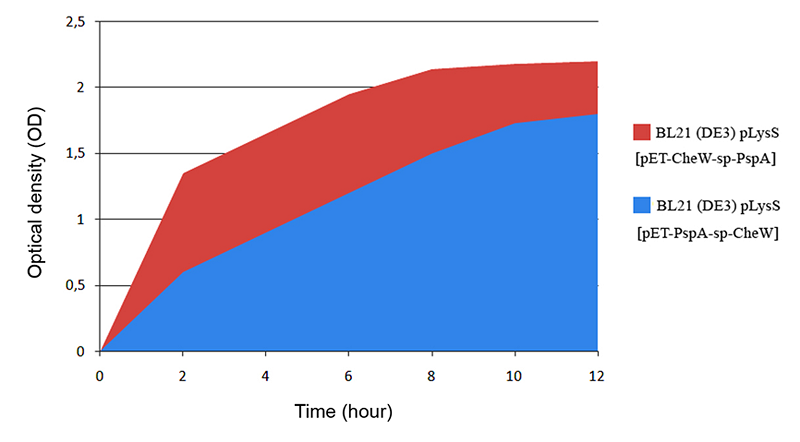
Figure 3.
Comparison of growth dynamics of E. coli BL21(DE3) pLysS cells prosucing PspA-sp-CheW and CheW-sp-PspA chimeric proteins
|
FUNDING
The research was supported by the Russian Science Foundation grant No. 23-15-00149.
REFERENCES
- O’Brien, K.L., Wolfson, L.J., Watt, J.P., Henkle, E., Deloria-Knoll, M., McCall, N., Lee, E., Mulholland, K., Levine, O.S., Cherian T., Hib and Pneumococcal Global Burden of Disease Study Team (2009) Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet, 374(9693), 893–902. DOI
- Goulart, C., Darrieux, M., Rodriguez, D., Pimenta, F.C., Brandileone, M.C., de Andrade, A.L., Leite, L.C. (2011) Selection of family 1 PspA molecules capable of inducing broad-ranging cross-reactivity by complement deposition and opsonophagocytosis by murine peritoneal cells. Vaccine, 29(8), 1634–1642. DOI
- Dagan, R., Melamed, R., Zamir, O., Leroy, O. (1997) Safety and imunnogenicity of tetravalent pneumococcal vaccines containing 6B, 14, 19F and 23F polysaccharides conjugated to either tetanus toxoid or diphtheria toxoid in young infants and their booster ability by native polysaccharide antigens. Pediatr. Infect. Dis. J., 16(11), 1053–1059. DOI
- Briles, D.E., Ades, E., Paton, J.C., Sampson, J.S., Carlone, G.M., Huebner, R.C., Virolainen, A., Swiatlo, E., Hollingshead, S. (2000) Intranasal immunization of mice with a mixture of the pneumococcal proteins PsaA and PspA is highly protective against nasopharyngeal carriage of Streptococcus pneumoniae. Infect. Immun., 68(2), 796–800. DOI
- Briles, D.E., Hollingshead, S., Brooks-Walter, A., Nabors, G.S., Ferguson, L., Schilling, M., Gravenstein, S., Braun, P., King, J., Swift, A. (2000) The potential to use PspA and other pneumococcal proteins to elicit protection against pneumococcal infection. Vaccine, 18(16), 1707–1711. DOI
- Wizemann, T.M., Heinrichs, J.H., Adamou, J.E., Erwin, A.L., Kunsch, C., Choi, G.H., Barash, S.C., Rosen, C.A., Masure, H.R., Tuomanen, E., Gayle, A., Brewah, Y.A., Walsh, W., Barren, P., Lathigra, R., Hanson, M., Langermann, S., Johnson, S., Koenig, S. (2001) Use of a whole genome approach to identify vaccine molecules affording protection against Streptococcus pneumoniae infection. Infect. Immun., 69(3), 1593–1598. DOI
- Converso, T.R., Goulart, C., Rodriguez, D., Darrieux, M., Leite, L.C.C. (2017) Rational selection of broadly cross-reactive family 2 PspA molecules for inclusion in chimeric pneumococcal vaccines. Microb. Pathog., 109, 233–238. DOI
- Hollingshead, S.K., Baril, L., Ferro, S., King, J., Coan, P., Briles, D.E. (2006) The Pneumococcal Proteins Epi Study Group. Pneumococcal surface protein A (PspA) family distribution among clinical isolates from adults over 50 years of age collected in seven countries. J. Med. Microbiol., 55(2), 215–221. DOI
- Daniels, C.C., Coan, P., King, J., Hale, J., Benton, K.A., Briles, D.E., Hollingshead, S.K. (2010) The proline-rich region of pneumococcal surface proteins A and C contains surface-accessible epitopes common to all pneumococci and elicits antibody-mediated protection against sepsis. Infect Immun., 78(5), 2163–2172. DOI
- Kono, M., Hotomi, M., Hollingshead, S.K., Briles, D.E., Yamanaka, N. (2011) Maternal immunization with pneumococcal surface protein A protects against pneumococcal infections among derived offspring. PLoS ONE, 6(10), e27102. DOI
- Roche, H., Håkansson, A., Hollingshead, S.K., Briles, D.E. (2003) Regions of PspA/EF3296 best able to elicit protection against Streptococcus pneumoniae in a murine infection model. Infect Immun., 71(3), 1033–1041. DOI
- Briles, D.E., King, J.D., Gray, M.A., McDaniel, L.S., Swiatlo, E., Benton, K.A. (1996) PspA, a protection-eliciting pneumococcal protein: Immunogenicity of isolated native PspA in mice. Vaccine, 14(9), 858–867. DOI
- da Costa Rodrigues, T., Zorzete, P., Miyaji, E.N., Gonçalves, V.M. (2024) Novel method for production and purification of untagged pneumococcal surface protein A from clade 1. Appl. Microbiol. Biotechnol., 108(1), 281. DOI
- Briles, D.E., Hollingshead, S.K., Swiatlo, E., Brooks-Walter, A., Szalai, A., Virolainen, A., McDaniel, L.S., Benton, K.A., White, P., Prellner, K., Hermansson, A., Aerts, P.C., van Dijk, H., Crain, M.J. (1997) PspA and PspC: Their potential for use as pneumococcal vaccines. Microb. Drug Resist., 3(4), 401-408. DOI
- Yadav, D.K., Yadav, N., Yadav, S., Haque, S., Tuteja, N. (2016) An insight into fusion technology aiding efficient recombinant protein production for functional proteomics. Arch. Biochem. Biophys., 612, 57–77. DOI
- Costa, S., Almeida, A., Castro, A., Domingues, L. (2014) Fusion tags for protein solubility, purification and immunogenicity in Escherichia coli: The novel Fh8 system. Front. Microbiol., 5, 63. DOI
- Ki, M.R., Pack, S.P. (2020) Fusion tags to enhance heterologous protein expression. Appl Microbiol. Biotechnol., 104(6), 2411–2425. DOI
- Grishin, D.V., Samoilenko, V.A., Gladilina, Y.A., Zhdanov, D.D., Pokrovskaya, M.V., Aleksandrova, S.S., Pokrovsky, V.S., Sokolov, N.N. (2019) Effect of heterologous expression of chemotaxis proteins from genus Thermotoga on the growth kinetics of Escherichia coli cells. Bull. Exper. Biol. Med., 167(3), 375–379. DOI
- Baker, M.D., Wolanin, P.M., Stock, J.B. (2006) Signal transduction in bacterial chemotaxis. Bioessays, 28(1), 9–22. DOI
- Randić, M., Pisanski, T. (2015) Protein alignment: Exact versus approximate. An illustration. J. Comput. Chem., 36(14), 1069–1074. DOI
- Larkin, M.A., Blackshields, G., Brown, N.P., Chenna, R., McGettigan, P.A., McWilliam, H., Valentin, F., Wallace, I.M., Wilm, A., Lopez, R., Thompson, J.D., Gibson, T.J., Higgins, D.G. (2007) Clustal W and Clustal X version 2.0. Bioinformatics, 23(21), 2947–2948. DOI
- Drury, L. (1996) Transformation of bacteria by electroporation. Methods Mol. Biol., 58, 249–256. DOI
- Delaney, S., Murphy, R., Walsh, F. (2018) A comparison of methods for the extraction of plasmids capable of conferring antibiotic resistance in a human pathogen from complex broiler cecal samples. Front. Microbiol., 9, 1731. DOI
- Gibson, D.G. (2011) Enzymatic assembly of overlapping DNA fragments. Methods Enzymol., 498, 349–361. DOI
- van Rosmalen, M., Krom, M., Merkx, M. (2017) Tuning the flexibility of glycine-serine linkers to allow rational design of multidomain proteins. Biochemistry, 56(50), 6565–6574. DOI