Alignment and Normalization of Mass Spectrometry Data Using the Hydrophobicity Index
Institute of Biomedical Chemistry, 10 Pogodinskaya str., Moscow, 119121 Russia; *e-mail: an.voronina@list.ru
Keywords: retention time; mass spectrometry; data alignment
DOI:10.18097/BMCRM00245
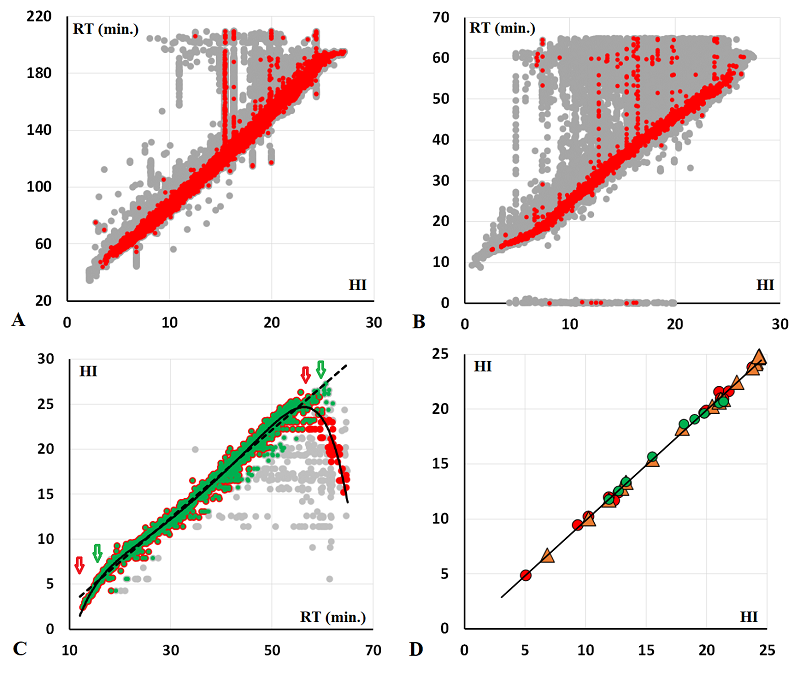
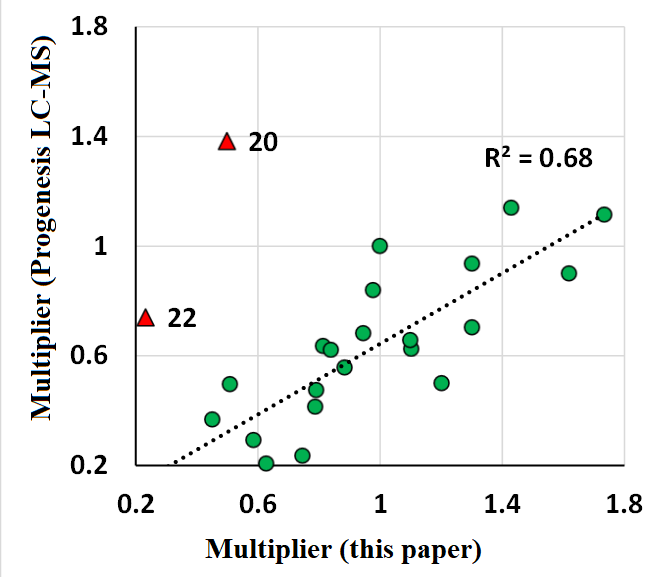
This paper presents a program for the alignment of data from mass spectrometry experiments by retention time on a chromatographic column. The program uses the experimentally obtained data set as a reference against which the alignment procedure is performed. The primary advantage of this approach consists in its capacity to align data sets that had significant variations in both peptide composition and substance amount, such as individual fractions derived from multivariate separation. To illustrate this, two datasets were employed. The first dataset contains data obtained after multivariate separation, while the second dataset exhibited comparable peptide composition across all samples. The second dataset was used to assess the efficacy of the alignment program in normalizing signal intensity between individual samples. The results were compared with the normalization results obtained by the Progenesis LC-MS program. The normalization multipliers obtained for 22 of the 24 samples exhibited good correlation with those calculated by the Progenesis LC-MS (R2 = 0.68). The program is freely available at http://lpcit.ibmc.msk.ru/AlignRT.

|
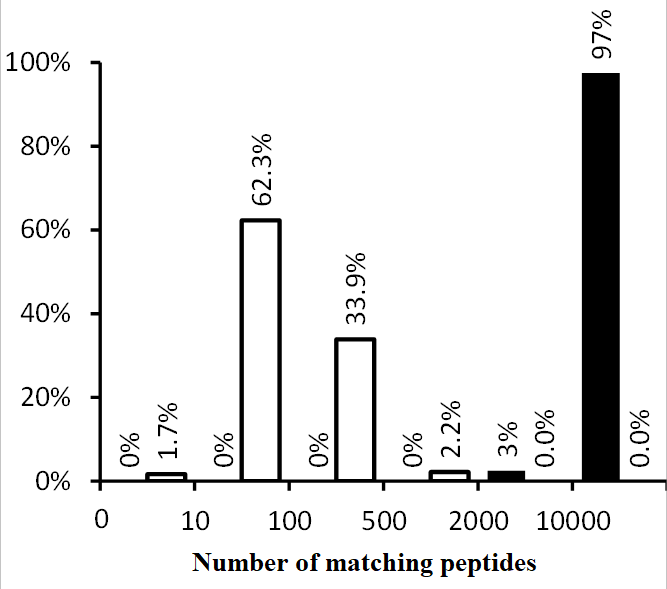
Figure 1.
The distribution of the number of matching peptides in pairwise comparisons of samples from sets 1 (black) and 2 (white).
|

|
Figure 4.
The comparison of multipliers for normalization of abundance calculated in this paper with data obtained in [17]. Samples 20 and 22, falling out of the general trend, are marked in red.
|
|
CLOSE

|
Table 1.
The number of peptides obtained by virtual cleavage by trypsin that could theoretically match the “reference” set for different biological species.
|
FUNDING
The work was performed within the framework of the Program for Basic Research in the Russian Federation for a long-term period (2021-2030) (№ 122030100170-5).
REFERENCES
- Lange, E., Tautenhahn, R., Neumann, S., Gröpl, C. (2008) Critical assessment of alignment procedures for LC-MS proteomics and metabolomics measurements. BMC Bioinformatics, 9, 375. DOI
- Ong, S.E., Mann, M. (2005) Mass spectrometry-based proteomics turns quantitative. Nature Chemical Biology, 1(5), 252-262. DOI
- Ong, S.E., Foster, L.J., Mann, M. (2003) Mass spectrometric-based approaches in quantitative proteomics. Methods (San Diego, Calif.), 29(2), 124-130. DOI
- Vandenbogaert, M., Li-Thiao-Té, S., Kaltenbach, H.M., Zhang, R., Aittokallio, T., Schwikowski, B. (2008) Alignment of LC-MS images, with applications to biomarker discovery and protein identification. Proteomics, 8(4), 650-672. DOI
- America, A.H., Cordewener, J.H. (2008) Comparative LC-MS: a landscape of peaks and valleys. Proteomics, 8(4), 731-749. DOI
- Fischer, B., Grossmann, J., Roth, V., Gruissem, W., Baginsky, S., Buhmann, J.M. (2006) Semi-supervised LC/MS alignment for differential proteomics. Bioinformatics, 22(14), e132-140. DOI
- Jaffe, J.D., Mani, D.R., Leptos, K.C., Church, G.M., Gillette, M.A., Carr, S.A. (2006) PEPPeR, a platform for experimental proteomic pattern recognition. Molecular & Cellular Proteomics, 5(10), 1927-1941. DOI
- Prince, J.T., Marcotte, E.M. (2006) Chromatographic alignment of ESI-LC-MS proteomics data sets by ordered bijective interpolated warping. Analytical Chemistry, 78(17), 6140-6152. DOI
- Ramiro, L., Faura, J., Simats, A., García-Rodríguez, P., Ma, F., Martín, L., Canals, F., Rosell, A., Montaner, J. (2023) Influence of sex, age and diabetes on brain transcriptome and proteome modifications following cerebral ischemia. BMC Neuroscience, 24(1), 7. DOI
- ProteomeXchange, project PXD051750. Retrieved January 29, 2025, from: http://central.proteomexchange.org/cgi/GetDataset?ID=PXD051750 DOI
- Branca, R., Orre, L., Johansson, H., Granholm, V., Huss, M., Pérez-Bercoff, A., Forshed, J., Käll, L., Lehtiö, J. (2014) HiRIEF LC-MS enables deep proteome coverage and unbiased proteogenomics. Nature Methods, 11, 59-62. DOI
- Xin, L., Qiao, R., Chen, X., Tran, H., Pan, S., Rabinoviz, S., Bian, H., He, X., Morse, B., Shan, B., Li, M. (2022) A streamlined platform for analyzing tera-scale DDA and DIA mass spectrometry data enables highly sensitive immunopeptidomics. Nature Communications, 13, 3108. DOI
- Wilburn, D.B., Shannon, A.E., Spicer, V., Richards, A.L., Yeung, D., Swaney, D.L., Krokhin, O.V., Searle, B.C. (2023) Deep learning from harmonized peptide libraries enables retention time prediction of diverse post translational modifications, bioRxiv, 5(30), 542978. DOI
- Wilhelm, M., Zolg, D.P., Graber, M., Gessulat, S., Schmidt, T., Schnatbaum, K., Schwencke-Westphal, C., Seifert, P., de Andrade Krätzig, N., Zerweck, J., Knaute, T., Bräunlein, E., Samaras, P., Lautenbacher, L., Klaeger, S., Wenschuh, H., Rad, R., Delanghe, B., Huhmer, A., Carr, S.A., Clauser, K.R., Krackhardt, A.M., Reimer, U., Kuster, B. (2021) Deep learning boosts sensitivity of mass spectrometry-based immunopeptidomics. Nature Communications, 12, 3346. DOI
- Gessulat, S., Schmidt, T., Zolg, D.P., Samaras, P., Schnatbaum, K., Zerweck, J., Knaute, T., Rechenberger, J., Delanghe, B., Huhmer, A., Reimer, U., Ehrlich, H.C., Aiche, S., Kuster, B., Wilhelm, M. (2019) Prosit: proteome-wide prediction of peptide tandem mass spectra by deep learning. Nature Methods, 16(6), 509-518. DOI
- Voronina, A.I., Rybina, A.V. (2023) A Program for Predicting the Retention Time of Peptides with Post-Translational Modifications. Biomedical Chemistry: Research and Methods, 6(3), e00196. DOI
- Rybina, A.V. (2024) Identification of mouse brain proteoforms: comparison of 2D-electrophoresis data and independent experiment with mass spectrometric identification. Biomeditsinskaya Khimiya, 70(6), 475-480. DOI
- Progenesis LC-MS version 4.0, Nonlinear Dynamics, Newcastle upon Tyne, UK.