The 40th Anniversary of the Institute of Physiologically Active Compounds of the Russian Academy of Sciences
Investigation of the Esterase Status as a Complex Biomarker of Exposure to Organophosphorus Compounds
1Institute of Physiologically Active Compounds of the Russian Academy of Sciences, 1 Severny proezd, Moscow region, Chernogolovka, 142432 Russia,*e-mail: gmakh@ipac.ac.ru 2Departments of Environmental Health Sciences and Neurology, University of Michigan, Ann Arbor, MI 48109 USA
Key words: acetylcholinesterase (AChE); butyrylcholinesterase (BChE); neuropathy target esterase (NTE); carboxylesterase (CaE); blood; biomarker; organophosphorus compounds (OPC)
DOI: 10.18097/BMCRM00028
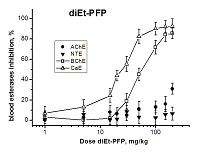
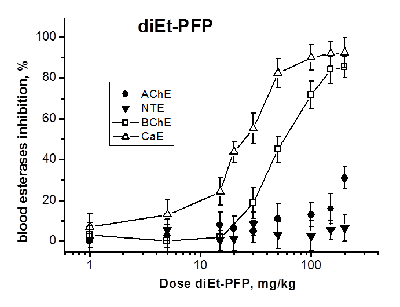
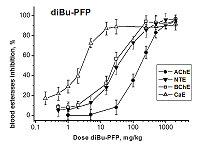
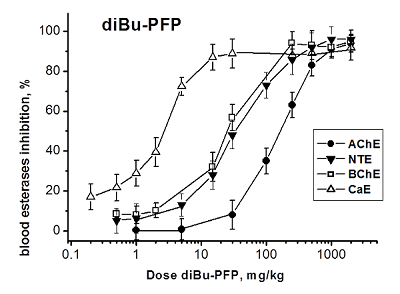
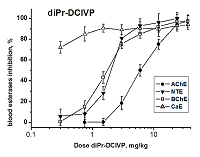
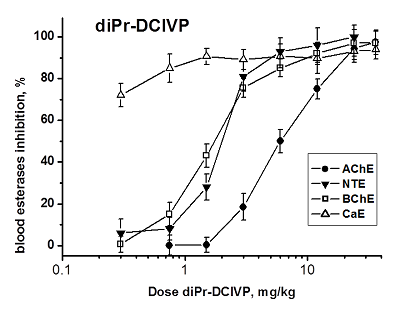
Development of biomarkers of human exposures to organophosphorus compounds OPCs and their quantification is a vital component of a system of prediction and early diagnostics of OPC-induced diseases. Our study was focused on investigation of esterase status as a complex biomarker of exposure to OPCs and an aid in accurate diagnosis. We suggest that this complex biomarker should be more effective and informative than standard assays of plasma butyrylcholinesterase (BChE), erythrocyte acetylcholinesterase (RBC AChE), and lymphocyte neuropathy target esterase (NTE). It will help: 1) to assess an exposure as such and to confirm the nonexposure of individuals suspected to have been exposed; 2) to determine if the exposure was to agents expected to produce acute and/or delayed neurotoxicity; 3) to perform dosimetry of the exposure, which provides valuable information for medical treatment. To confirm this hypothesis, we have examined the changes in activity of blood AChE, NTE, BChE and carboxylesterase (CaE) 1 h after i.p. administration of increasing doses of three OPCs with different esterase profiles: the known neuropathic compound O,O-dipropyl-O-dichlorovinyl phosphate (C3H 7O)2P(O)OCH=CCl2 (diPr-DClVP) as the control compound and two model dialkylphosphates (C2H5O)2P(O)OCH(CF3)2 (diEt-PFP) and (C4H9O)2P(O)OCH(CF3)2 (diBu-PFP). The esterases assay was performed in hemolysed blood by spectrophotometric (AChE, BChE, CaE) and biosensor (NTE) methods. Analysis of the obtained dose-dependences for blood esterases inhibition showed that blood BChE and CaE were the most sensitive biomarkers, allowing detection of low doses. Inhibition of blood NTE and AChE can be used to assess the likelihood that an exposure to OPC would produce cholinergic and/or delayed neuropathic effects.
|
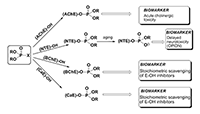
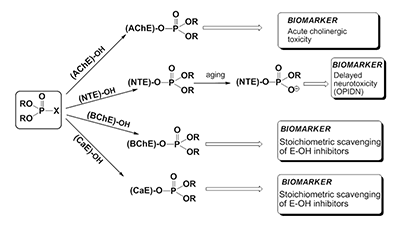
Figure 1.
OPC interaction with various serine esterases (E-OH), their possible toxic effects, the role in mechanisms of toxicity and function as biomarkers.
|
|
CLOSE

|
Table 1.
In vitro inhibitor activity (ki, M-1min-1) of dialkylphosphates diEt-PFP, diBu-PFP
and diPr-DClVP against individual enzymes AChE, NTE, BChE, CaE (esterase profile of OPCs) |
|
CLOSE

|
Table 2.
Inhibitor selectivity of diEt-PFP, diBu-PFP and diPr-DClVP to individual enzymes AChE, NTE, BChE, CaE and acute toxicity of the compounds
|
|
CLOSE

|
Table 3.
Median effective doses (ED50, mg/kg) for diEt-PFP, diBu-PFP and diPr-DClVP as esterases inhibitors in mouse blood 1h after i.p. compounds administrations
|
ACKNOWLEDGEMENTS
This work was supported by the NATO Science for Peace and Security Program (grant № SfP 984082) and by Russian State assignment № 0090-2017-0019. The authors thank to the colleagues from the Laboratory of molecular toxicology of IPAC RAS O.G. Serebryakova and T.G. Galenko for assistance in toxicological experiments and work with animals.
REFERENCES
- Costa, L. G. (1996). Biomarker research in neurotoxicology: the role of mechanistic studies to bridge the gap between the laboratory and epidemiological investigations. Environmental Health Perspectives, 104 Suppl 1, 55-67.
- National Research Council, N. (1987). Biological markers in environmental health research. Environmental Health Perspectives, 74, 3-9.
- Makhaeva, G. F., Radchenko, E. V., Palyulin, V. A., Rudakova, E. V., Aksinenko, A. Y., Sokolov, V. B., Zefirov, N. S., & Richardson, R. J. (2013). Organophosphorus compound esterase profiles as predictors of therapeutic and toxic effects. Chemico-Biological Interactions, 203(1), 231-237. DOI
- Richardson, R. J., Hein, N. D., Wijeyesakere, S. J., Fink, J. K., & Makhaeva, G. F. (2013). Neuropathy target esterase (NTE): overview and future. Chemico-Biological Interactions, 203(1), 238-244. DOI
- Richardson, R. J., & Makhaeva, G. F. (2014). Organophosphorus Compounds. In P. Wexler (Ed.), Encyclopedia of Toxicology (3rd edition ed., Vol. 3, pp. 714-719): Elsevier Inc., Academic Press.
- Richardson, R. J., Worden, R. M., Wijeyesakere, S. J., Hein, N. D., Fink, J. K., & Makhaeva, G. F. (2015). Neuropathy Target Esterase as a Biomarker and Biosensor of Delayed Neuropathic Agents. 935-952. DOI
- Lotti, M., & Johnson, M. K. (1978). Neurotoxicity of organophosphorus pesticides: predictions can be based on in vitro studies with hen and human enzymes. Archives of Toxicology, 41(3), 215-221. DOI
- Richardson, R. J. (2005). Organophosphate Poisoning, Delayed Neurotoxicity In P. Wexler (Ed.), Encyclopedia of Toxicology (Second ed., Vol. 3, pp. 302-306). Oxford: Elsevier.
- Richardson, R. J., Worden, R. M., & Makhaeva, G. F. (2009). Biomarkers and Biosensors of Delayed Neuropathic Agents. In R. C. Gupta (Ed.), Handbook of the Toxicology of Chemical Warfare Agents (pp. 859-876): Elsevier.
- Johnson, M. K. (1982). The target for initiation of delayed neurotoxicity by organophosphorus esters: Biochemical studies and toxicological applications. In J. R. B. E. Hodgson, R.M. Philpot (Ed.), Reviews in Biochemical Toxicology (Vol. 4, pp. 141–212). Amsterdam: Elsevier.
- Thompson, C. M., & Richardson, R. J. (2005). Anticholinesterase Insecticides. In T. C. Marrs & B. Ballantyne (Eds.), Pesticide Toxicology and International Regulation (Current Toxicology Series) (pp.89 - 127). John Wiley & Sons, Ltd.
- Makhaeva, G. F., Iankovskaia, V. L., Kovaleva, N. V., Fetisov, V. I., Malygin, V. V., Torgasheva, N. A., & Khaskin, B. A. (1999). [O,O-dialkyl-S-bromomethylthiophosphates--inhibitors of mammalian choline- and carboxyl esterases: structure-activity relationship]. Bioorganicheskaia Khimiia, 25(1), 3-7.
- Jokanovic, M. (2001). Biotransformation of organophosphorus compounds. Toxicology, 166(3), 139-160.
- Masson, P., & Lockridge, O. (2010). Butyrylcholinesterase for protection from organophosphorus poisons: catalytic complexities and hysteretic behavior. Archives of Biochemistry and Biophysics, 494(2), 107-120. DOI
- Casida, J. E., & Quistad, G. B. (2004). Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chemical Research in Toxicology, 17, 983-998. DOI
- Peeples, E. S., Schopfer, L. M., Duysen, E. G., Spaulding, R., Voelker, T., Thompson, C. M., & Lockridge, O. (2005). Albumin, a new biomarker of organophosphorus toxicant exposure, identified by mass spectrometry. Toxicological Sciences, 83(2), 303-312. DOI
- Tarhoni, M. H., Lister, T., Ray, D. E., & Carter, W. G. (2008). Albumin binding as a potential biomarker of exposure to moderately low levels of organophosphorus pesticides. Biomarkers, 13(4), 343-363. DOI
- Bajgar, J. (1992). Biological monitoring of exposure to nerve agents. British Journal of Industrial Medicine, 49, 648-653.
- Bertoncin, D., Russolo, A., Caroldi, S., & Lotti, M. (1985). Neuropathy target esterase in human lymphocytes. Archives of Environmental Health, 40(3), 139-144. DOI
- Dudek, B. R., & Richardson, R. J. (1982). Evidence for the existence of neurotoxic esterase in neuronal and lymphatic tissue of the adult hen. Biochemical Pharmacology, 31, 1117-1121.
- Maroni, M., & Bleecker, M. L. (1986). Neuropathy target esterase in human lymphocytes and platelets. Journal of Applied Toxicology, 6(1), 1-7.
- Richardson, R. J., & Dudek, B. R. (1983). Neurotoxic esterase: Characterization and potential for a predictive screen for exposure to neuropathic organophosphates. In P. C. K. J. Miyamoto (Ed.), Pesticide Chemistry: Human Welfare and the Environment (Vol. 3, pp. 491-495). Oxford: Pergamon
- Lotti, M. (1986). Biological monitoring for organophosphate-induced delayed polyneuropathy. Toxicology Letters, 33(1-3), 167-172. DOI
- Lotti, M., Becker, C. E., Aminoff, M. J., Woodrow, J. E., Seiber, J. N., Talcott, R. E., & Richardson, R. J. (1983). Occupational exposure to the cotton defoliants DEF and merphos. A rational approach to monitoring organophosphorous-induced delayed neurotoxicity. Journal of Occupational Medicine, 25(7), 517-522.
- Lotti, M., Moretto, A., Zoppellari, R., Dainese, R., Rizzuto, N., & Barusco, G. (1986). Inhibition of lymphocytic neuropathy target esterase predicts the development of organophosphate-induced delayed polyneuropathy. Archives of Toxicology, 59(3), 176-179. DOI
- Schwab, B. W., & Richardson, R. J. (1986). Lymphocyte and brain neurotoxic esterase: Dose and time dependence of inhibition in the hen examined with three organophosphorus esters. Toxicology and Applied Pharmacology, 83(1), 1-9. DOI
- Lotti, M. (1987). Organophosphate-induced delayed polyneuropathy in humans: Perspectives for biomonitoring. Trends in Pharmacological Sciences, 81, 176-177.
- Sigolaeva, L. V., Makower, A., Eremenko, A. V., Makhaeva, G. F., Malygin, V. V., Kurochkin, I. N., & Scheller, F. W. (2001). Bioelectrochemical analysis of neuropathy target esterase activity in blood. Analytical Biochemistry, 290(1), 1-9. DOI
- Makhaeva, G. F., Sigolaeva, L. V., Zhuravleva, L. V., Eremenko, A. V., Kurochkin, I. N., Malygin, V. V., & Richardson, R. J. (2003). Biosensor detection of Neuropathy Target Esterase in whole blood as a biomarker of exposure to neuropathic organophosphorus compounds. Journal of Toxicology and Environmental Health, Part A, 66, 599-610. DOI
- Sokolovskaya, L. G., Sigolaeva, L. V., Eremenko, A. V., Gachok, I. V., Makhaeva, G. F., Strakhova, N. N., Malygin, V. V., Richardson, R. J., & Kurochkin, I. N. (2005). Improved electrochemical analysis of neuropathy target esterase activity by a tyrosinase carbon paste electrode modified by 1-methoxyphenazine methosulfate. Biotechnology Letters, 27(16), 1211-1218. DOI
- Makhaeva, G. F., Malygin, V. V., Strakhova, N. N., Sigolaeva, L. V., Sokolovskaya, L. G., Eremenko, A. V., Kurochkin, I. N., & Richardson, R. J. (2007). Biosensor assay of neuropathy target esterase in whole blood as a new approach to OPIDN risk assessment: review of progress. Human & Experimental Toxicology, 26(4), 273-282. DOI
- Wilson, B. W., & Henderson, J. D. (1992). Blood esterase determinations as markers of exposure. Reviews of Environmental Contamination and Toxicology, 128, 55-69.
- Richardson, R. J. (1995). Assessment of the neurotoxic potential of chlorpyrifos relative to other organophosphorus compounds: a critical review of the literature. Journal of Toxicology and Environmental Health, 44(2), 135-165. DOI
- Lockridge, O., & Schopfer, L. M. (2006). Biomarkers of organophosphate exposure . In R. C. Gupta (Ed.), Toxicology of Organophosphate & Carbamate Compounds (pp. 703-711).Burlington: Academic Press.
- Costa, L. G., Cole, T. B., Jarvik, G. P., & Furlong, C. E. (2003). Functional genomic of the paraoxonase (PON1) polymorphisms: effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annual Review of Medicine, 54, 371-392. DOI
- Sokolovskaya, L. G., Sigolaeva, L. V., Eremenko, A. V., Kurochkin, I. N., Makhaeva, G. F., Malygin, V. V., Zykova, I. E., Kholstov, V. I., & Zavyalova, N. V. (2004). Family of biosenor analyzers for assessment of “esterase status” of organism. Russian Chemical Journal, 1-2(13-14), 21-31.
- Makhaeva, G., Rudakova, E., Boltneva, N., Sigolaeva, L., Eremenko, A., Kurochkin, I., & Richardson, R. (2009). Blood Esterases as a Complex Biomarker for Exposure to Organophosphorus Compounds. In C. Dishovsky & A. Pivovarov (Eds.), Counteraction to Chemical and Biological Terrorism in East European Countries (pp. 177-194): Springer Netherlands.
- Rudakova, E. V., Boltneva, N. P., & Makhaeva, G. F. (2011). Comparative analysis of esterase activities of human, mouse, and rat blood. Bulletin of Experimental Biology and Medicine, 152(1), 73-75.
- Kurdyukov, I. D., Shmurak, V. I., Nadeyev, A. D., Voitenko, N. G., Prokofyeva, D. S., & Goncharov, N. V. (2012). "Esteruse status" of the organism at exposure to toxic substances and pharmaceutical preparations. Toxicological Reviews (Russian), 6(117), 6-13.
- Maroni, M., Colosio, C., Ferioli, A., & Fait, A. (2000). Biological Monitoring of Pesticide Exposure: a review. Toxicology, 143(1), 5-118. DOI
- Albert, J. R., & Stearns, S. M. (1974). Delayed neurotoxic potential of a series of alkyl esters of 2, 2-dichlorovinyl phosphoric acid in the chicken. Toxicology and Applied Pharmacology, 29(1), 136.
- Rudakova, E. V., Makhaeva, G. F., & Sigolaeva, L. V. (2014). Investigation of Mice Blood Neuropathy Target Esterase as Biochemical Marker of Exposure to Neuropathic Organophosphorus Compounds. In J. R. S. C. Dishovsky (Ed.), Toxicological Problems (pp. 39-50). Sofia, Bulgaria: Military Publishing House.
- Makhaeva, G. F., Serebryakova, O. G., Boltneva, N. P., Galenko, T. G., Aksinenko, A. Y., Sokolov, V. B., & Martynov, I. V. (2008). Esterase profile and analysis of structure-inhibitor selectivity relationships for homologous phosphorylated 1-hydroperfluoroisopropanols. Doklady Biochemistry and Biophysics, 423(1), 352-357. DOI
- Rudakova, E. V., Makhaeva, G. F., Galenko, T. G., Aksinenko, A. Y., Sokolov, V. B., & Martynov, I. V. (2013). A new selective inhibitor of mouse blood plasma carboxylesterase. Doklady Biochemistry and Biophysics, 449, 87-89. DOI
- Sigolaeva, L., Makhaeva, G., Rudakova, E., Boltneva, N., Porus, M., Dubacheva, G., Eremenko, A., Kurochkin, I., & Richardson, R. J. (2010). Biosensor analysis of blood esterases for organophosphorus compounds exposure assessment: Approaches to simultaneous determination of several esterases. Chemico-Biological Interactions, 187(1-3), 312-317. DOI
- Sigolaeva, L. V., Dubacheva, G. V., Porus, M. V., Eremenko, A. V., Rudakova, E. V., Makhaeva, G. F., Richardson, R. J., & Kurochkin, I. N. (2013). A layer-by-layer tyrosinase biosensor for assay of carboxylesterase and neuropathy target esterase activities in blood. Analytical Methods, 5(16), 3872. DOI
- Rudakova, E. V., Serebryakova, O. G., Boltneva, N. P., Galenko, T. G., & Makhaeva, G. F. (2012). A biochemical model in mice for assessment of neuropathic potential of organophosphorus compounds. Toxicological Reviews (Russian), 6, 20-24.
- Makhaeva, G. F., Rudakova, E. V., Sigolaeva, L. V., Kurochkin, I. N., & Richardson, R. J. (2016). Neuropathy target esterase in mouse whole blood as a biomarker of exposure to neuropathic organophosphorus compounds. Journal of Applied Toxicology, 36(11), 1468-1475. DOI
- Makhaeva, G. F., Aksinenko, A. Y., Sokolov, V. B., Serebryakova, O. G., & Richardson, R. J. (2009). Synthesis of organophosphates with fluorine-containing leaving groups as serine esterase inhibitors with potential for Alzheimer disease therapeutics. Bioorganic & Medicinal Chemistry Letters, 19(19), 5528-5530. DOI
- Makhaeva, G. F., & Malygin, V. V. (1999). A stable preparation of hen brain neuropathy target esterase for rapid biochemical assessment of neurotoxic potential of organophosphates. Chemico-Biological Interactions, 119–120, 551-557. DOI
- Ellman, G. L., Courtney, K. D., Andres, V., & Feather-Stone, R. M. (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology, 7, 88-95.DOI
- Johnson, M. K. (1977). Improved assay of neurotoxic esterase for screening organophosphates for delayed neurotoxicity potential. Archives of Toxicology, 37(2), 113-115. DOI
- Aldridge, W. N., & Reiner, E. (1969). Acetylcholinesterase. Two types of inhibition by an organophosphorus compound: one the formation of phosphorylated enzyme and the other analogous to inhibition by substrate. Biochemical Journal, 115(2), 147-162.
- Padilla, S., Lassiter, T. L., & Hunter, D. (1999). Neurodegeneration Methods and Protocols. In H. A. T. J.Harry (Ed.), Methods in Molecular Medicine (Vol. 22, pp. 237-245). N.J. Totowa: Humana Press Inc.
- Reiner, E., Bosak, A., & Simeon-Rudolf, V. (2004). Activity of cholinesterases in human whole blood measured with acetylthiocholine as substrate and ethopropazine as selective inhibitor of plasma butyrylcholinesterase. Archives of Industrial Hygiene and Toxicology, 55(1), 1-4.
- Worek, F., Mast, U., Kiderlen, D., Diepold, C., & Eyer, P. (1999). Improved determination of acetylcholinesterase activity in human whole blood. Clinica Chimica Acta, 288(1-2), 73-90. DOI
- De Vriese, C., Gregoire, F., Lema-Kisoka, R., Waelbroeck, M., Robberecht, P., & Delporte, C. (2004). Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology, 145(11), 4997-5005. DOI
- Huang, T. L., Shiotsuki, T., Uematsu, T., Borhan, B., Li, Q. X., & Hammock, B. D. (1996). Structure-activity relationships for substrates and inhibitors of mammalian liver microsomal carboxylesterases. Pharmaceutical Research, 13(10), 1495-1500.
- Chanda, S. M., Mortensen, S. R., Moser, V. C., & Padilla, S. (1997). Tissue-specific effects of chlorpyrifos on carboxylesterase and cholinesterase activity in adult rats: An in vitro and in vivo comparison. Fundamental and Applied Toxicology, 38, 148-157.
- Sigolaeva, L. V., Pergushov, D. V., Synatschke, C. V., Wolf, A., Dewald, I., Kurochkin, I. N., Fery, A., & M?ller, A. H. E. (2013). Co-assemblies of micelle-forming diblock copolymers and enzymes on graphite substrate for an improved design of biosensor systems. Soft Matter, 9(10), 2858-2868. DOI